For more than four decades I’ve been involved in the dietary supplement business, from my humble beginnings working in a natural foods store to creating my own line of the highest quality supplements available on the market.
It has been a long, challenging, and educational road. And I understand—from an insider’s viewpoint—just how difficult it is to know whom you can trust when it comes to choosing the most effective dietary supplements.
How I Ended Up Creating Dietary Supplements
I never really thought I would be in the dietary supplement business. My journey started in the mid-1970s when I worked at a natural foods store while still in high school, which evolved into becoming part owner of a well-known natural foods store in Connecticut 10 years later. In 1989, as an herbalist and nutritionist, I established my first clinic, Centre for Natural Healing, in Connecticut. At that time, I also began creating formulas for several well-known herbal companies.

As these companies grew, I saw them move more toward commercialization, taking on investors, and eventually selling their companies to large corporations. Unfortunately, this evolution typically does not bode well for the purity and efficacy of products, because the ‘bottom line’ encourages the use of the least expensive raw materials. For the most part, I lost my faith in these companies.
I had no intention of starting my own line of products. But in 2000, I developed several herbal and nutritional formulas for my clinic. After meeting with the owner of a company who advised changing the ingredients from the highest quality to inferior alternatives, I realized that I would have to do it myself. This was the birth of Natura Health Products.
What Makes a High-Quality Herbal Medicine?
Consumers increasingly rely on botanical products for self-medicating, because these products are generally considered safe and are associated with fewer side effects than synthetic drugs. However, the enormous variety and variability of products presents a tremendous challenge for both consumers and health-care professionals. The quality of a plant extract is strictly related to the quality of the botanical source and the method of preparation. In addition, the quality and composition of the active components can vary according to several factors, including plant species, the method of cultivation, age, and the part of the plant used.1

There is now a substantial amount of data on the occurrence of adulteration, the market situation, and its subsequent consequences on the industry and end users simply for the unethical financial gain of the seller.2
Key factors for a high-quality herbal medicine:
- Genetic factors (correct and specific species)
- Climate
- Soil characteristics (pH, fertilization, heavy metals)
- Infections from pests and microbes (exposure activates the plants’ immune response, creating various compounds)
- Harvest time
- Parts of the plant used
- Duration of time from when the herb is harvested to extraction
- Processing (extraction, solvent polarity, temperature, duration, distillation, fermentation, purification)
- Storage (light, oxygen, humidity, temperature)
- Extraction solvents used
- Concentration of active constituents
- Amount of herbal extract and active constituents per serving
Comparison of standardized products with reproducible chemical composition best assures reproducible pharmacologic activity. The content of active markers in commercial herbal products can vary considerably.
Additional Factors to Consider in Herbal and Nutritional Supplements
Considering the source of the raw material is essential in evaluating the quality of herbal and nutritional supplements. Although a company may begin with good quality raw materials, they might dilute the supplement with less expensive raw materials. This is a common practice with supplements, as well as with foods. For example, much of what is sold as pure olive oil is actually a mix of olive oil with cheaper and less healthful oils.

This same approach is widely used in the dietary supplement industry. Why? Because they need to be competitive in the marketplace and need to have a large profit margin. Shareholder profits often drive a company, while more ethical reasons—such as helping people and the planet—take a back seat. A large percentage of dietary supplements also contain pharmaceuticals, according to a recent JAMA study.
“Active pharmaceuticals continue to be identified in 67.9% of dietary supplements tested, especially those marketed for sexual enhancement or weight loss, even after FDA warnings.”
From 2007 through 2016, the FDA identified 776 adulterated dietary supplements, and 146 different dietary supplement companies were implicated. Most of these products were marketed for sexual enhancement, weight loss, or muscle building, with 157 adulterated products containing more than one unapproved ingredient. The most common adulterants were sildenafil for sexual enhancement supplements, sibutramine for weight loss supplements, and synthetic steroids or steroid-like ingredients for muscle building supplements. In some cases, despite warnings, the FDA found the products continued to be adulterated. In recent years (2014-2016), 117 of 303 adulterated samples were identified through online sampling and 104 of 303 were identified through the examination of international mail shipments.3
Many dietary supplements available to consumers in the marketplace may be contaminated or substituted with alternative plant species and fillers that are not listed on the labels. The World Health Organization has stated that the adulteration of herbal products is a threat to consumer safety.
A 2013 study reported in the BMC Medicine journal found that “59% of the products contained plant species not listed on the labels and most commercial herb products including adaptogens lack active constituents.”5
According to the World Health Organization, “Standardization is the process of prescribing a set of standards or inherent characteristics, constant parameters, definitive qualitative and quantitative values that carry an assurance of quality, efficacy, safety, and reproducibility.” Only products fulfilling the basic requirements of quality have reproducible safety and efficacy profiles.5-7
Adaptogens in the Marketplace: What are You Actually Getting?
The substitution or mixing of medicinal grade adaptogens is very common. According to a study published in the February 2018 Journal of Pharmaceutical and Biomedical Analysis, DNA barcoding methods tested 25 Siberian ginseng and 14 rhodiola (R. rosea) products which are widely available to UK customers to test whether the herbal ingredient stated on the label is also in the product. All Eleutherococcus (E) senticosus supplements contained E. senticosus, however, 36% also contained an eleutherococcus species other than E. senticosus. In three out of the 13 rhodiola products that produced amplifiable DNA, the researchers found only DNA sequences matching alfalfa (declared on the product label) and fenugreek (not declared). In the other 10 supplements rhodiola was detected but only five matched the target species R. rosea.8
The Problems with Panax Ginseng

The root of Panax ginseng has been used medicinally for thousands of years in China, Korea, and Japan for prophylactic and curative properties in cases of decreased physical and mental capacities including tiredness, exhaustion, weakness, and during convalescence. However, the quality of ginseng and other adaptogenic plants is a major problem and has been for some time. The composition and quality of ginseng products on the market is highly variable. This presents a challenge for both consumers and health-care professionals searching for high-quality, reliable ginseng products that have a proven safety and efficacy profile.
When ginseng is administered orally, a significant fraction of ginsenosides are metabolized by intestinal microflora into compound K, which is considered to be the main constituent with pharmacological effects.9 Botanical extract standardization is of crucial importance in this context as it determines the reproducibility of the quality of the product essential for the evaluation of effectiveness and safety.10
Russian researchers found it very difficult to get a regular supply of Panax ginseng yielding consistent active compounds. This was one of the major reasons for the shift from Panax ginseng to Eleutherococcus as a primary general adaptogen. Also, the fact that Panax costs more than Eleuthero, and Panax in certain individuals causes mild but pronounced side effects, mostly nervousness.
Researchers testing the purity and potency of ginseng products found that they vary tremendously, as reported in the June issue of the American Journal of Clinical Nutrition. According to an analysis of 25 commercial ginseng preparations found in a health food store, the actual concentration of active compounds differed significantly from the amounts listed on labels. Furthermore, concentrations of active ingredients in ginseng preparations varied 15- to 200-fold in capsule and liquid products.
Of the 25 samples, only 11 (44%) were labeled as containing a specific concentration of ginsenosides, the active compounds. Of these, five (45%) contained more than the labeled amount and six (55%) contained less than the specified amount.11
Adulteration of Ashwagandha (Withania somnifera) Extracts

Ashwagandha is a primary adaptogen and is widely used as a general energy-increasing tonic known to promote learning and memory, and in geriatric problems. The root of the plant has been traditionally used to promote youthful vigor, endurance, strength, health, and for increasing the production of vital fluids, muscle fat, blood, lymph, semen, and cells.
The adulteration of ashwagandha root extract by adding undeclared extracts from aerial parts of the plant to commercial products provides less value to the consumer and adversely affects the reputation of botanicals and natural products industry. This practice has a considerable negative effect on companies that sell genuine root material and extract, because of the availability of lower-cost adulterated materials and extracts. Validated analytical methods that enable detection of this type of adulteration are available and should be adopted in every quality control laboratory. The implementation of such validated methods by ethical suppliers and botanical product manufacturers will provide appropriate testing data, as opposed to non-specific spectrophotometric methods that often provide misleading results with regard to the proper identity and authenticity of ashwagandha root raw materials and extracts.12
Medical Mushrooms: Most are Not What You Expect

Medicinal mushrooms, which are gaining in popularity, are often found with little to no active constituents, do not meet label claims, or are being promoted based on total polysaccharide content and not beta-glucans. Beta-glucans make up the majority of the fungal cell wall and are responsible for the immunological and health promoting activity of medicinal mushrooms.
In the mushroom world, companies and producers of mycelium are claiming that their products have all of the fungal parts: mycelium, mushroom, and spores. Producers of mycelium grow their mycelium on cooked, sterilized cereal grain (rice, oats, sorghum, etc.) and they separate the grain from the final product. Thus, it is not 100% mycelium like often assumed, but a myceliated grain/starch that has little beta glucans, and a high percentage of alpha glucans. Mushrooms contain very low levels of alpha-glucans in the form of glycogen. They do not contain starch.
Beta-glucan taken orally differs from other food substances. This type of glucan is acid resistant, so it passes through the stomach virtually unchanged. Macrophages in the mucous lining of the intestinal wall pick up the beta glucan particles through beta glucan receptors. Immediate activation of these cells follows, and they are later able to travel back to the local lymph nodes as part of their natural antigen-presenting function, to release cytokines and induce systematic immune activation. β-glucans taken orally may lower blood cholesterol by preventing the absorption of cholesterol from food in the stomach and intestines.
There are several beta-glucan supplement products that claim β-glucans taken by mouth can only be absorbed if the product is prepared by a special patented process that ‘micronizes’ β-glucan particles to a size of one micron or less. However, there is no reliable evidence to support such a claim.”13
A good example of the huge discrepancy in the marketplace is the well-known reishi (Ganoderma lingzhi, formally, lucidum) mushroom extract.

In one study, eleven commercial reishi extract products were tested for bioactive compounds, including polysaccharides and triterpenes. None of them contained even one-half the level of triterpenes that correlate with the mushroom extract used in clinical trials (>4% tripterpenes), and only a few contained sufficient polysaccharides (>10% polysaccharides).14
Delving Deeper into the Science of Medicinal Mushrooms
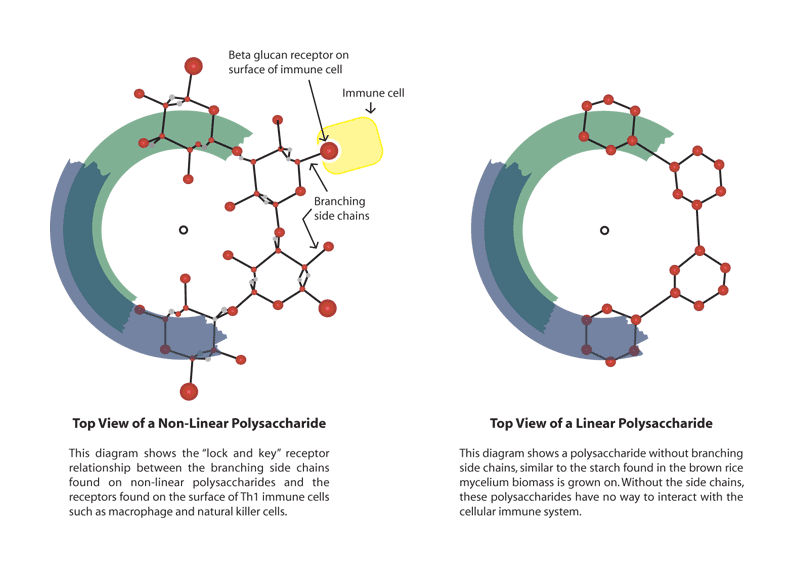
Beta-glucans from fungi constitute a heterogeneous group of glucose polymers, consisting of a backbone of Beta-(1,3)–linked beta-Dglucopyranosyl units with Beta-(1, 6) linked side chains of varying distributions and lengths. As beta-glucans are not found in animals, they enhance and modulate the immune system and induce innate immune responses, which protect us from attack by pathogenic microbes.
The immunomodulatory effects of beta-glucans are known to be inconsistent and variable, probably due to differences in the degree of branching, polymer length, and tertiary structures among beta-glucans. Certain glucans, including zymosan and lentinan, appear to efficiently activate phagocytes. Whereas neutrophils are effective against pyogenic bacteria, NK cells circulate in blood to lyse cancer and virus-infected cells. In addition, beta-glucans stimulate macrophages to produce cytokines, and these in turn activate adaptive immunity against foreign antigens, which involves both B and T cells. B cells produce antibodies to mediate humoral immunity, whereas T cells induce cell-mediated immunity.
The adaptive immune response also involves dendritic cells (DCs) derived from monocytes, and these present antigens to T cells for activation of immune responses. There are several reports indicating that DCs are functionally defective in tumor-bearing host. Beta-glucans have been shown to enhance the antigen presenting function of dendritic cells, thereby inducing tumor-specific cytotoxic T cells.15
Adulteration of Saw Palmetto (Serenoa repens)

Environmental concerns, including the issue of overharvesting, affect the amount of raw materials available for processing. For example, in recent years saw palmetto fruits have not been in abundant supply since environmental conditions (including heavy rains) and the new state requirement for harvesting permits have adversely affected their availability. Manufacturers need to be particularly vigilant in years when the saw palmetto harvest is low, and in subsequent years when possible adulterated materials may more likely be found in the marketplace.
To ensure quality control, saw palmetto extract ingredient specifications should be developed in a manner that will ensure the absence of adulterants. Quality control analysts should be informed of the appropriate tests that can be used to verify the authenticity of the material. Establishing the country of origin for a shipment of saw palmetto berries may reduce the likelihood of a substitute species being used, as authentic saw palmetto grows only in a small range in the southeast U.S. 16
Serious Concerns about Synthetic Turmeric/Curcumin Adulteration

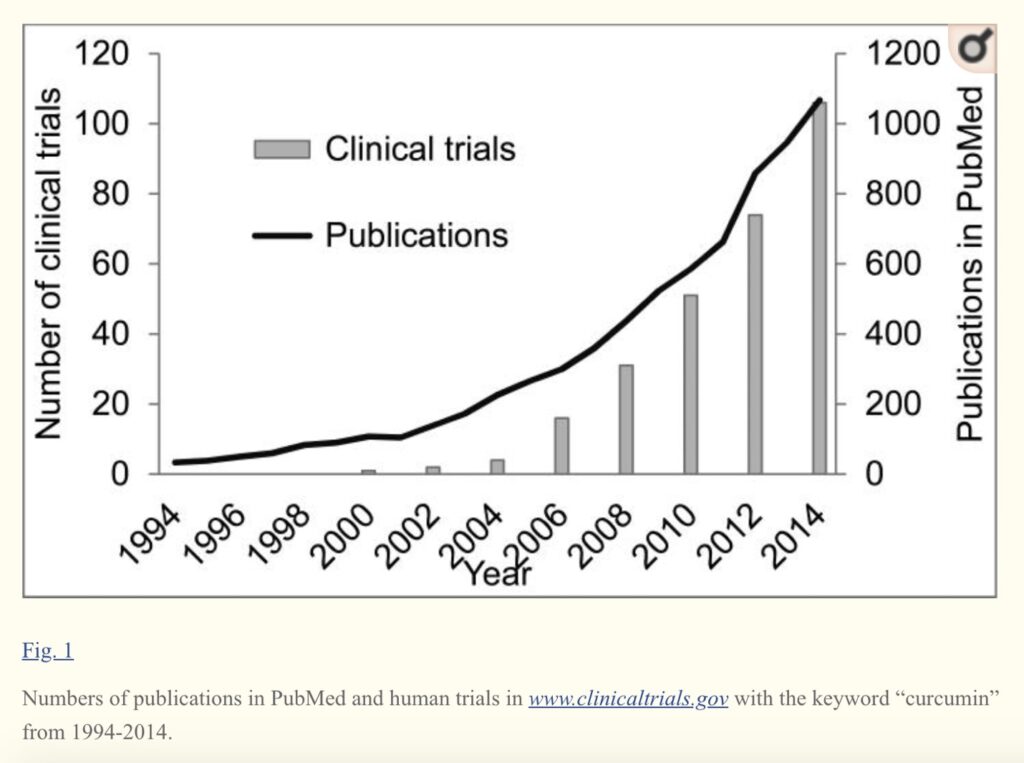
A recent analysis of 87 different turmeric dietary supplements highlights the wide range
of available products and the challenges this presents to consumers. The analysis also raises concerns regarding product adulteration with synthetic curcumin.
Scientists from the University of Arizona and Vanderbilt University reported that over 80% of 87 products tested (which mostly contained turmeric‐derived curcuminoid extracts) were only within 20% of the labeled amounts.
Their data indicated that 94% of the products contained turmeric‐derived curcuminoid extracts (the rest were turmeric root only), and these were combined with other bioactives in 47% of products. The main additive was piperine, which can enhance bioavailability.
The majority of products contained curcuminoid at levels that were within 80% of the label claims, but the composition of curcumin relative to dimethoxy- and bisdemethoxycurcumin (the three curcuminoids that exist naturally together) did not match USP criteria in almost 60% of the products. Importantly, 16% of products contained curcumin at over 90% of total curcuminoids.17
Many companies use marketing gimmicks touting their form of herb as superior in terms of bioavailability. But this is misleading, for several reasons: 1) Bioavailability or absorption does not mean better activity, or better clinical results. 2) Looking for plant compounds in the blood as a marker for bioavailability is clinically irrelevant. Medicinal herbs and their compounds undergo complex biotransformation and/or fermentation into numerous metabolites, which are actually what is active and translatable.
The Bioactive Effects of Curcumin
Curcumin and other curcuminoids from Curcuma longa (turmeric) are important bioactive compounds exhibiting various pharmacological activities. The active components of turmeric, collectively known as curcuminoids, are among the most promising natural compounds currently being studied. Curcumin is the main bioactive ingredient in turmeric extract and is widely consumed as part of the spice mix curry or as a dietary supplement.
Clinical studies have demonstrated the role of curcumin in the management of various chronic health conditions. These studies also increase our understanding of how curcumin is metabolized.
On ingestion, curcumin forms several active metabolites undergoing phase I metabolism such as dihydrocurcumin, tetrahydrocurcumin, hexahydrocurcumin and octahydrocurcumin.
In course of its metabolism inside the body, curcumin also forms degradation compounds such as ferulic acid and bicyclopentadione. While these phase I metabolites have been shown to have beneficial biological activity, compounds such as curcumin glucuronides and sulfates formed after phase II metabolism have proven ineffective in independent studies.18
In Summary: Trusting Your Herbal and Nutritional Products
According to the World Health Organization, most herbal products tested are of poor quality, including considerable product substitution, adulteration, contamination and use of fillers.19
When you see an herb listed as a 5:1 or 10:1 or 30:1, whatever the extraction ratio is, it doesn’t take into account the quality of the herb, whether or not it has been adulterated, or if the active isolated compound was removed prior to the extraction process. Unfortunately, this is not an uncommon practice. If in the quest to make a profit, manufacturers shop for bargains in the raw materials they use, their end product will be of poor quality, and the consumer will suffer the consequences.
That brings me to the question: Whom do you trust, and why?

Working in clinical practice for more than three decades, I am committed to using the best for those I serve. I use the highest quality ingredients, those that are validated by science and/or tradition from the best and most trusted raw material companies in the world, of which there are only a few. I believe that those of us in the healthcare field—whether in clinical practice or involved in manufacturing dietary and herbal supplements—are entrusted with the health of our clients.
It is our responsibility to uphold the highest standard, and to keep our focus on the well being of those we serve, not financial gain.
References:
- Bilia AR, Bergonzi MC, The G115 standardized ginseng extract: an example for safety, efficacy, and quality of an herbal medicine, Journal of Ginseng Research, https://doi.org/10.1016/j.jgr.2019.06.003.
- Upton R, Petrone C, Swisher D. American Herbal Pharmacopoeia: Ashwagandha Root, Withania somnifera: Analytical, Quality Control, and Therapeutic Monograph. Scotts Valley, CA: American Herbal Pharmacopoeia, 2000.
- Jenna Tucker, MPH; Tessa Fischer, DVM, MPH; Laurence Upjohn, PharmD; David Mazzera, PhD; Madhur Kumar,MS, PhD, Unapproved Pharmaceutical Ingredients Included in Dietary Supplements Associated With US Food and Drug Administration Warnings, JAMA Netw Open. 2018;1(6):e183337. doi:10.1001/jamanetworkopen.2018.3337.
- Steven G et. al. DNA barcoding detects contamination and substitution in North American herbal products, BMC Medicine, October 2013 DOI: 10.1186/1741-7015-11-222.
- World Health Organization, Quality assurance of pharmaceuticals: a compendium of guidelines and related materials. Good manufacturing practices and inspection, vol. 2, WHO, Geneva (1996).
- World Health Organization Guidelines for the assessment of herbal medicines, WHO, Geneva (1996), European Medicines Agency Guidelines on quality of herbal medicinal product/traditional herbal medicinal products, EMA, London (2011)
- World Health Organization General guidelines for methodologies on research and evaluation of traditional medicine, WHO, Geneva (2000).
- Ruhsam M., Hollingsworth., PM., Authentication of Eleutherococcus and Rhodiola herbal supplement Products in the United Kingdom Journal of Pharmaceutical and Biomedical Analysis, 2018 Feb 5;149:403409. doi: 10.1016/j.jpba.2017.11.025. Epub 2017 Nov 9.
- Bilia AR, Bergonzi MC, The G115 standardized ginseng extract: an example for safety, efficacy, and quality of an herbal medicine, Journal of Ginseng Research, https://doi.org/10.1016/j.jgr.2019.06.003.
- Anna R. Bilia Maria C.Bergonzi, The G115 standardized ginseng extract: an example for safety, efficacy, and quality of an herbal medicine, Journal of Ginseng Research, https://doi.org/10.1016/j.jgr.2019.06.003.
- Harkey MR, Henderson GL, Gershwin ME, Stern JS, Hackman RM. Variability in commercial ginseng products: an analysis of 25 preparations. American Journal of Clinical Nutrition 2001; 73(6): 1101-1106.
- Singh VK, Mundkinajeddu D, Agarwal A, Nguyen J, Sudberg S, Gafner S, Blumenthal M. Adulteration of ashwagandha (Withania somnifera ) roots and extracts. Botanical Adulterants Prevention Bulletin . Austin, TX: ABC-AHP-NCNPR Botanical Adulterants Prevention Program; 2018.
- Sandeep Rahar, Gaurav Swami,1 Navneet Nagpal, Manisha A. Nagpal, and Gagan Shah Singh, Preparation, characterization, and biological properties of β-glucans, J Adv Pharm Technol Res. 2011 Apr-Jun; 2(2): 94–103., doi: 10.4103/2231-4040.82953.
- S. Wachtel-Galor, Herbal Medicine: Biomolecular and Clinical Aspects. Chapter 9 Ganoderma lucidum (Lingzhi or Reishi) A Medicinal Mushroom 2nd edition. Boca Raton (FL): CRC Press; 2011.)
- Kenji Ina1,Takae Kataoka, and Takafumi Ando, The Use of Lentinan for Treating Gastric Cancer, Anti-Cancer Agents in Medicinal Chemistry, 2013, 13, 681-688 681
- M.B. Skiba et al., “Curcuminoid Content and Safety‐Related Markers of Quality of Turmeric Dietary Supplements Sold in an Urban Retail Marketplace in the United States”Molecular Nutrition & Food Research Published online ahead of print, doi: 10.1002/mnfr.201800143
- Gafner S, Baggett S. Adulteration of saw palmetto (Serenoa repens ). Version 3. Botanical Adulterants Prevention Bulletin . Austin, TX: ABC-AHP-NCNPR Botanical Adulterants Prevention Program. 2018.
- Muhammed Majeed, Kalyanam, Nagabhushanam, Reductive Metabolites of Curcuminoids, NutraScience Publications, 1st addition, 2019, Pg. 3-9.
- Steven G Newmaster1*, Meghan Grguric2, Dhivya Shanmughanandhan3, Sathishkumar Ramalingam and Subramanyam Ragupathy, DNA barcoding detects contamination and substitution in North American herbal products, Newmaster et al. BMC Medicine 2013, 11:222 http://www.biomedcentral.com/1741-7015/11/222