I have always been a proponent of medical freedom and believe that individuals should weigh the risk-to-benefit ratio when deciding anything when it comes to modern medicine. Unfortunately, so much of what we’re led to believe about the safety of the novel coronavirus vaccine is false and mis-leading. Although there many reputable doctors and scientists willing to voice concerns and openly debate the risks, their voices are not being heard by the majority of the public and their professional careers and reputations are being threatened and often smeared, distorted, and censored by the media. It is more important than ever to have truth and transparency when it comes to new technologies in medicine.
Traditional vaccines present the entire virus in a live-attenuated form (measles, mumps, rubella, varicella, rotavirus, Sabin oral poliovirus, yellow fever, and some influenza vaccines) or an inactivated form (Salk poliovirus, hepatitis A, rabies, and some other influenza vaccines), leading to a polyclonal response to not just one, but a number of viral proteins. This multiplicity of humoral and T cell responses probably explains why no convincing vaccine escape strains have been documented for these viruses; with the exception to this being the influenza virus.[1] The mRNA vaccines for COVID-19 however, are engineered in an entirely different way, with a far more complex design and effect.
The messenger RNA technology platform being researched and developed for the past two decades for gene therapy to target diseases such as cancer and Alzheimer’s (and fraught with challenges and limitations), has rapidly evolved in the emerging field of nucleic acid therapeutics thanks to the revolutionary advancement of nanotechnology or nanomedicine. In recent years, numerous scientific breakthrough discoveries, patents, and licensing agreements led to drug manufacturers being readily positioned to enter the race to produce the COVID-19 vaccines at an unprecedented scale in response to the pandemic with great reliance on the availability of lipid nanoparticles (LNPs). The ability to utilize the mRNA platform for vaccine delivery as a result of the lipid nanoparticle technology has drawn tremendous attention and provided a great deal of hope with regards to a solution to the COVID-19 pandemic around the world (at least the parts of the world that had the financial resources to afford it). However, the widespread, general use of nanotechnology in the mass population (and the inability to reduce disease transmission) raises many ethical issues, especially when it comes to vaccine mandates, political interference in health policy, and a lack of accountability in the case of vaccine injuries.
Moderna, Pfizer /BioNTech, CureVac and Arcturus have all developed mRNA-based vaccines for COVID-19 combining mRNA technology with lipid nanoparticle technology to deploy a sophisticated delivery system designed to achieve a specific biological response. A lipid nanoparticle must be used to deliver the mRNA to the cells to avoid mRNA degradation, which makes it a key aspect of the vaccine’s efficacy. After the mRNA is delivered to a cell, it instructs the cell to produce the SARS-CoV-2 spike protein, thereby eliciting an immune response.[2]
This technology made a fast pace of discovery and manufacturing possible, critical features that could be fully utilized in a biotech and pharmaceutical setting and, by the way, be extremely profitable, especially when you add in the ongoing need for what appears to be endless boosters.[3] “Senior executives from China’s CanSino Biologics and early investors in Moderna have also become billionaires on paper as shares skyrocketed, partly in expectation of profits earned from Covid vaccines, which also bode well for the companies’ future prospects.” Covid-19 vaccines have created at least nine new billionaires after shares in companies producing the shots soared.[4],[5]
What makes this new technology so attractive is that the production of mRNA vaccines can take only days or weeks to complete, as opposed to the production of attenuated or inactivated viruses.[6] It can be made by in vitro transcription of mRNA, where virtually any mRNA sequence can be produced from a DNA template. Further, an mRNA vaccine would provide the cell with the direct instructions for expressing an immunogenic protein of interest via cytoplasmic translation.[7]
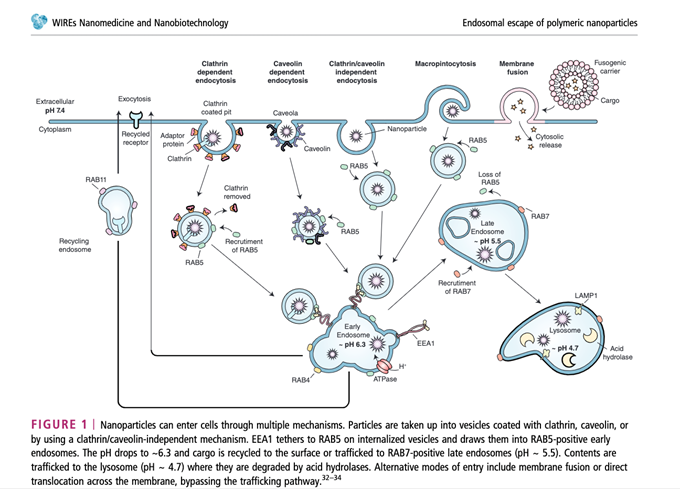
We have been led to believe that this technology has been around for a very long time and that it has been extensively studied and proven safe. However, the race to drug discovery and development has far outpaced the ability to assess the impact of nanoparticles on the cellular environment in humans, especially for widespread, repeat delivery of the spike protein to the general population over the long-term. With other nucleic acid-based therapeutic modalities, there have been issues with the delivery system and this has led to delays in the emergence of this technology with use to date. One of the issues that occurs is the RNA molecule begins to degrade rapidly in the body by ribonucleases (enzymes) or become entrapped by endosomes before reaching the site.[8] The next issue is that the negatively charged phosphodiester backbone of an RNA makes it difficult to cross biological membranes.[9],[10] Also, muscle cells are not very efficient in the translation of the mRNA encoding the Spike protein.[11]
So, this created a huge problem and basically up until the modifications made to the COVID-19 mRNA vaccines, other vaccines candidates were even less effective. The solution to this conundrum was to use lipid nanoparticles to protect the RNA until it reached the target site so now it would no longer degrade. Both the Pfizer-BioNTech and Moderna mRNA vaccines use LNPs to encapsulate the mRNA genetic material for more efficient cell delivery. In 2018, new generations of LNPs were used to deliver patisiran®, an RNAi-based drug, which generated optimism for RNA therapeutics delivery.[12]
Now, with LNPs, their precious cargo can be carried to the lymph nodes and from there, they are internalized by dendritic cells where the blueprint instructions synthesize the Spike protein. Once the protein inside the cell is encoded, it stimulates cellular and humoral immune responses (T and B cells) capable of identifying and destroying the virus that expresses this viral capsule protein.[13] The scientific community proclaims that without LNP formulations, the success of mRNA vaccines would not have been possible.
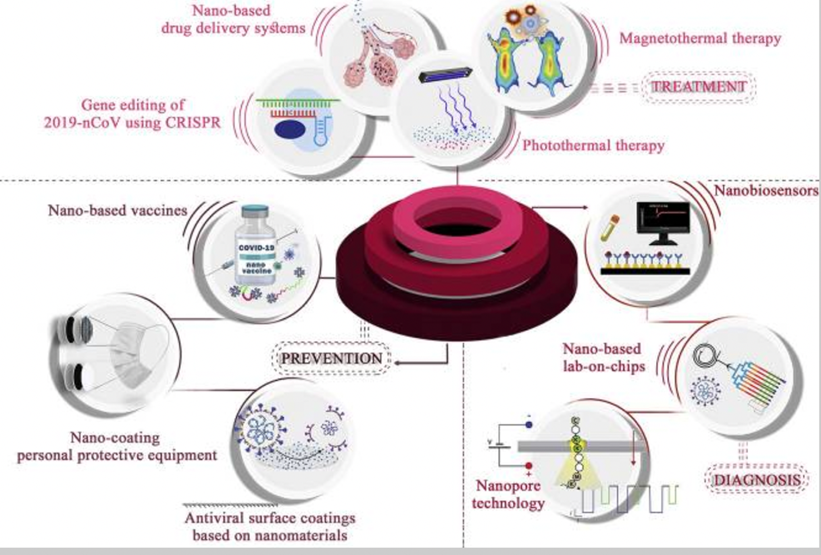
Schematic representation of nanotechnology in the battle against COVID-19. Nanotechnology can enhance the efficacy of existing therapeutics, preventive methods, and diagnostic strategies, as well as provide novel approaches.[14]
Unfortunately, when it comes to the COVID-19 vaccines, LNPs might deliver the mRNA to delicate cells or places that we don’t want them to, such as neurons in the brain or spinal cord. LNPs are often used to overcome the problem of the Blood Brain Barrier (BBB) blocking medical drugs from entering the brain.[15] Given that the BBB and blood-spinal cord barrier are lipid-soluble, the LNP-encapsulated mRNA vaccines, which are engineered using PEGylation (a coating that makes them “stealth liposomes” resulting in a longer circulating half-life as well as capable of evading certain aspects of the immune system) may be able to enter the nucleus of the cells in the brain and central nervous system.[16],[17]
Nanosystems have been designed to prevent the premature degradation of subunit vaccines and enhance their targeted delivery.[18] Nanocarriers such as poly(lactic-co-glycolic acid) (PLGA), approved by the United States Food and Drug Administration (FDA), along with polymeric nanoparticles and calcium phosphate, can be used for the delivery of immunogenic biomolecules.[19] Overall, liposomes, polysaccharide particles, dendrimers, and cationic nanoemulsions have all shown the potential to enhance the stability and delivery of mRNA-based vaccines;[20] however their long-term effects have not been studied.
By making many of these vaccine alterations, they were able to increase the production of neutralizing antibodies and to a lesser extent T cell responses, that are considered the gold standard for vaccine benefit.[21]
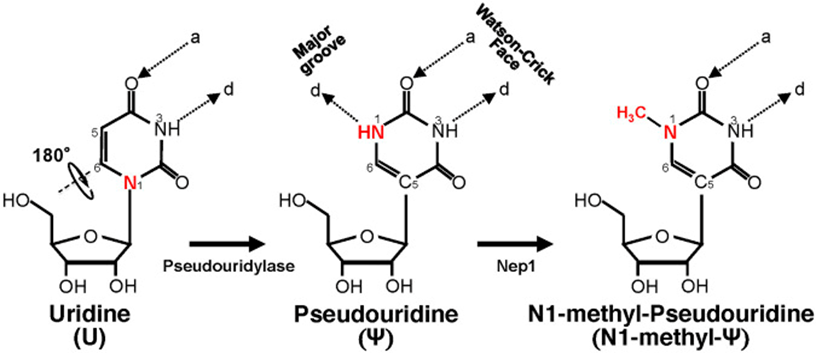
Another known drug delivery problem to overcome was that the in vitro transcribed (IVT) mRNA would be prone to nuclease degradation when injected into animals or humans and not reach the intended site. The IVT mRNA would also lead to innate immunogenicity (the immune system would over-activate) similar to what would happen when infected by a pathogen.[22] This exogenous nucleotide material can stimulate an innate immune response in organisms, generating an abnormal response with the potential to affect tissues other than the target cells of the therapy. To prevent this, nucleoside modifications are made to the mRNA (substituting N1-methyl-pseudouridine for uridine) to decrease this unwanted immune response.[23] Even with the modifications made, including efforts to reduce toxicity and increase tolerability, many of the adverse effects, including cardiomyopathy in young men, are a result of the increased immune response created by the vaccine. This activation of cells (Toll-like receptors, specifically TLR 3,7 & 8, and dendritic cells) exposed to mRNA can activate pro-inflammatory cascades which may have effects at the myocardial level.[24], [25]
Schematic representation of U-to-Ψ isomerization and additional N1 methylation. Ψ is a rotational isomer of uridine, in which the N-C glycosidic bond is substituted with the C-C bond. The isomerization reaction also creates an extra hydrogen bond donor (-N1H). Ψ can be further methylated at the N1 position by Nep1 (an N1-specific Ψ methyltransferase) to generate N-methyl-Ψ. d, hydrogen bond donor; a, hydrogen bond acceptor.[26]
With the substitution of the N1-methyl-pseudouridine, the vaccine’s ability to make the Spike protein dramatically increases. Depending on where the mRNA end up, the subsequent mRNA-induced spike protein expression can possibly trigger biological reactions we don’t want.
Sahin and colleagues initially developed mRNA vaccines with the aim of stimulating immunity against tumors. In those studies, the net negative charge of the mRNA lipid nanoparticle resulted in their macropinocytosis by splenic dendritic cells, activation of Toll-like receptor 7 (TLR7), production of interferon-α, and triggering of antigen-specific immunity.[27] In parallel, Kariko and colleagues developed a modification of mRNA-based vaccines that incorporated 1-methylpseudouridine (m1Ψ), in place of uridine, finding that m1Ψ mRNA had a higher translational capacity and lower innate immune activation because it did not stimulate TLR7.[28]
When naturally occurring modified nucleosides, for example, pseudouridine (Ψ), 5- methylcytidine (m5C), N6-methyladenosine (m6A), 5-methyluridine (m5U), or 2-thiouridine (s2U) were incorporated into the transcript, most of the TLRs were no longer activated, causing dysfunction within the innate immune system.[29],[30] This means with lower innate immune response there is less risk of an acute autoimmune/ inflammatory reaction from the vaccine, but in the long-term, an overall weakened effect on the immune system as a whole, particularly via downstream immune signaling pathways, DNA repair mechanisms, nuclear factor-kappa b activation, and regulation of apoptosis (programmed cell death).
Toll-like receptors (TLRs) are important mediators of inflammatory pathways in the gut, which play a major role in mediating the immune responses towards a wide variety of pathogen-derived ligands and link adaptive immunity with innate immunity. Numerous studies in different populations across the continents have reported on the significant roles of TLR gene polymorphisms in modulating the risk of various cancers.[31] TLRs play an important role in recognition of viral particles and activation of the innate immune system. Activation of TLR pathways leads to secretion of pro‐inflammatory cytokines, which are important to the first stage of the immune response and then downregulate as we move into subsequent stages.[32]
Another concern is that mRNA vaccines are leaky and don’t work well at adapting to new variants of SARS-CoV-2, which continue to evolve with resistance to immunity induced by recombinant spike protein vaccines (which are based on the original sequence, Wuhan-Hu-1).[33] According to the latest research, the vaccine’s effectiveness begins to wane within 1 month. A new study published in The New England Journal of Medicine[34] has focused on the relative effectiveness of the fourth dose, compared to the three dose vaccination regime, against confirmed infection and severe illness among older persons in the Israeli population.
So, for ongoing protection, assuming the vaccines are effective, you would need to get boosted every 2-3 months. As new spin off variants keep coming, there will be a constant need to reformulate the vaccines so that they better match the circulating strains. This is not feasible for many reasons, especially given that many poor countries around the world have less than 5% of their population vaccinated at all! Ironically, many of these countries have natural immunity and have done much better than the rich counties that have 90% + of their population fully vaccinated. An unresolved question is the affect that the variant mutations might have on T cell immunity. Substitutions occurring in T cell epitopes have the potential to impair cytotoxic T lymphocyte or T helper recognition, which might lead to delayed elimination of infected cells, or suboptimal help provided to B cells and the antibody response.[35]
The propaganda promoting the vaccines has significantly misrepresented how the immune system works and has led people to disregard the importance of T cell immunity. Vaccine manufacturers have been producing vaccines based solely on the production of neutralizing antibodies however, T cell responses against COVID-19 are often robust and create better immunity than antibody responses, though both are important. Antibody and B cell responses are relatively short lived and frequently became undetectable within 4 years, while T cell responses can be elicited after 17 years.[36],[37]
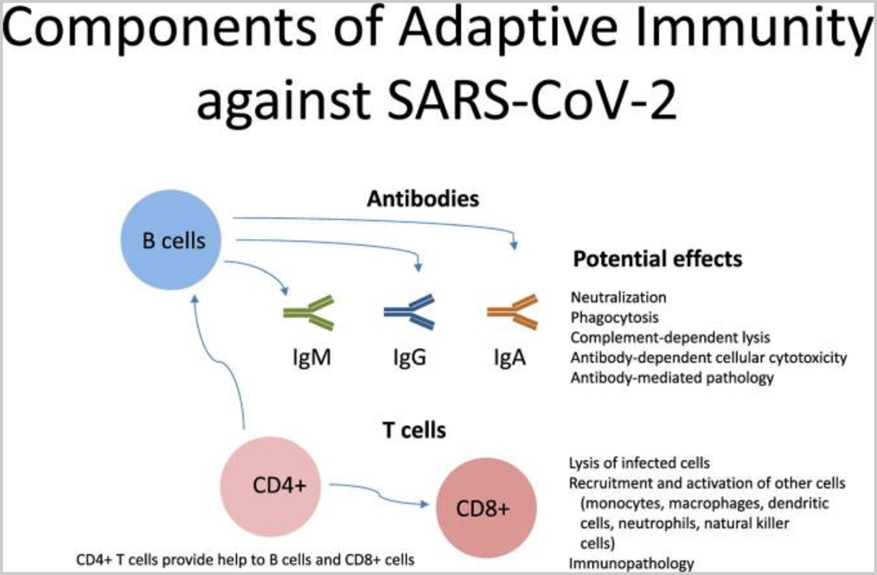
Ongoing research has confirmed that natural immunity of SARS-CoV-2 elicits broadly directed and functionally replete memory T cell responses, suggesting that natural exposure or infection may prevent recurrent episodes of severe COVID-19.[39]
“Nature is a totally efficient, self-regenerating system. If we discover
the laws that govern this system and live synergistically within them,
sustainability will follow and humankind will be a success.” – Buckminster Fuller
For those in good health, with well-functioning immune systems and none of the known risk factors that predispose certain individuals to severe adverse events from COVID-19, the use of herbal and nutritional medicine can effectively mitigate viral replication and clearance, as well as support and promote a controlled inflammatory response and a robust immune response.
Recently, the World Health Organization (WHO) held a meeting with 21 international experts to review and analyze the reports and randomized controlled trials of traditional Chinese medicine (TCM) in various phases of the progression of COVID-19 disease.
In order to emphasize the safety and efficacy of TCM in treating patients with COVID-19, Dr Zhang Weidong outlined the evidence that is readily available. He presented existing pooled data showing the following:
- 12 reliable RCTs were selected from a list of 71 for consideration by the Expert Panel, and there are reliable examples of real-world evidence among 79 cohort studies.
- For evidence on shortening the time to symptom resolution in patients with non-severe disease, there are seven RCTs with over 1000 patients.
- Evidence for slowing the rate of progress from non-severe to severe disease is supported by five RCTs and two real-world evidence studies with a total of 2179 patients.
- The time taken for nucleic acid conversion in patients with non-severe disease is reported in five RCTs with 826 patients.
- There are three RCTs and one real-world study with evidence of reduced hospital stays for non-severe patients, and one RCT and five real-world evidence studies (11 676 patients) to support the argument for reduced mortality in patients with severe disease.
- One RCT with 58 patients showed that a TCM product significantly reduces the rate of conversion of critical cases, the rate of mechanical ventilation and the length of ICU stay, as well as shortening the time to symptom resolution.
- TCM can reduce the use of resources and there is also evidence to suggest that TCM may help to prevent communicating the disease to close contacts. A study conducted in Yangzhou among 5686 persons in quarantine facilities found that the 3438 persons in the TCM treatment group had a positive infection rate of 0.29%, while the 2248 persons in the untreated group had a positive rate of 1.73%.
In summary, TCM had a role to play at all stages of viral infection, as follows:
- Prevention – during lockdown many people voluntarily chose to consume plants and herbs with medicinal value. They were encouraged to do so as such foods can help stimulate the immune system and promote health and wellness.
- Mild and moderate disease – the RCTs data indicates that TCM was seen to shorten the time to resolution of symptoms, viral clearance and to reduce the proportion of patients progressing to severe disease.
- Severe and critical disease – patients were treated with conventional medicine along with TCM. It was felt that this combined approach both shortened the length of stay in intensive care and reduced the duration of mechanical ventilation. It was claimed that TCM reduced mortality among critically ill patients.
- Convalescence – TCM serves as the main pillar of care at this stage. TCM interventions were reported to improve clinical symptoms and the quality of life.
The Chair proposed that these data should be published in an international journal to generate further discussion and analysis. Participants felt that there were sufficient data for the meeting to make a “cautiously optimistic” judgement that TCM could be helpful in the treatment of COVID-19.[40]
[1] Williams, Thomas C, and Wendy A Burgers. “SARS-CoV-2 evolution and vaccines: cause for concern?.” The Lancet. Respiratory medicine vol. 9,4 (2021): 333-335. doi:10.1016/S2213-2600(21)00075-8
[2] Gaviria M, Kilic B. A network analysis of COVID-19 mRNA vaccine patents. Nat Biotechnol. 2021 May;39(5):546-548. doi: 10.1038/s41587-021-00912-9. PMID: 33981074.
[3] Jackson N. A. C., Kester K. E., Casimiro D., Gurunathan S., DeRosa F. (2020). The Promise of mRNA Vaccines: a Biotech and Industrial Perspective. npj Vaccin. 5, 11. 10.1038/s41541-020-0159-8
[4] https://www.cnn.com/2021/05/21/business/covid-vaccine-billionaires/index.html, https://www.forbes.com/billionaires/
[5] Morais, Pedro et al. “The Critical Contribution of Pseudouridine to mRNA COVID-19 Vaccines.” Frontiers in cell and developmental biology vol. 9 789427. 4 Nov. 2021, doi:10.3389/fcell.2021.789427
[6] Pascolo S. (2021). Synthetic Messenger RNA-Based Vaccines: from Scorn to Hype. Viruses 13, 270. 10.3390/v13020270
[7] Krieg P. A., Melton D. A. (1984). Functional Messenger RNAs Are Produced by SP6in Vitrotranscription of Cloned cDNAs. Nucl. Acids Res. 12, 7057–7070. 10.1093/nar/12.18.7057
[8] Wadhwa A., Aljabbari A., Lokras A., Foged C., Thakur A. (2020). Opportunities and Challenges in the Delivery of mRNA-Based Vaccines. Pharmaceutics 12, 102. 10.3390/pharmaceutics12020102
[9] Dowdy SF. Overcoming cellular barriers for RNA therapeutics. Nat Biotechnol. 2017 Mar;35(3):222-229.
[10] Morais P, Adachi H, Yu YT. The Critical Contribution of Pseudouridine to mRNA COVID-19 Vaccines. Front Cell Dev Biol. 2021 Nov 4;9:789427.
[11] Rijkers GT, Weterings N, Obregon-Henao A, Lepolder M, Dutt TS, van Overveld FJ, Henao-Tamayo M. Antigen Presentation of mRNA-Based and Virus-Vectored SARS-CoV-2 Vaccines. Vaccines (Basel). 2021 Aug 3;9(8):848.
[12] Hoy S. M. (2018). Patisiran: First Global Approval. Drugs 78, 1625–1631. 10.1007/s40265-018-0983-6
[13] Ruffell D. (2021). The Future in an RNA Molecule: from mRNA Vaccines to Therapeutics – an Interview with Drew Weissman. FEBS Lett. 595, 2305–2309. 10.1002/1873-3468.14190
[14] Hasanzadeh, Akbar et al. “Nanotechnology against COVID-19: Immunization, diagnostic and therapeutic studies.” Journal of controlled release : official journal of the Controlled Release Society vol. 336 (2021): 354-374. doi:10.1016/j.jconrel.2021.06.036
[15] Ma, Feihe et al. “Neurotransmitter-derived lipidoids (NT-lipidoids) for enhanced brain delivery through intravenous injection.” Science advances vol. 6,30 eabb4429. 24 Jul. 2020, doi:10.1126/sciadv.abb4429
[16] Thomas O.S., Weber W., Overcoming physiological barriers to nanoparticle delivery – are we there yet? Front Bioeng Biotechnol. 2019; 7:415. doi: 10.3389/fbioe.2019.00415.
[17] Lombardo S.M., Schneider M., Tureli A.E., Tureli N.G., Key for crossing the BBB with nanoparticles: the rational design. Beilstein J. Nanotechnol. 2020, 11, 866-883.
[18] Peek L.J., Middaugh C.R., Berkland C. Nanotechnology in vaccine delivery. Adv. Drug Deliv. Rev. 2008;60(8):915–928.
[19] Hasanzadeh, Akbar et al. “Nanotechnology against COVID-19: Immunization, diagnostic and therapeutic studies.” Journal of controlled release : official journal of the Controlled Release Society vol. 336 (2021): 354-374. doi:10.1016/j.jconrel.2021.06.036
[20] Zeng C, Hou X, Yan J, Zhang C, Li W, Zhao W, Du S, Dong Y. Leveraging mRNA Sequences and Nanoparticles to Deliver SARS-CoV-2 Antigens In Vivo. Adv Mater. 2020 Oct;32(40):e2004452.
[21] Papadopoulos, Dimitris et al. “Predictive Factors for Neutralizing Antibody Levels Nine Months after Full Vaccination with BNT162b2: Results of a Machine Learning Analysis.” Biomedicines vol. 10,2 204. 18 Jan. 2022, doi:10.3390/biomedicines10020204
[22] Hoerr I., Obst R., Rammensee H.-G., Jung G. (2000). In Vivo application of RNA Leads to Induction of Specific Cytotoxic T Lymphocytes and Antibodies. Eur. J. Immunol. 30, 1–7. 10.1002/1521-4141(200001)30:1<1:aid-immu1>3.0.co;2-#
[23] Karikó K., Buckstein M., Ni H., Weissman D. Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008.
[24] Favere K., Bosman M., Klingel K., Heymans S., Van Linthout S., Delputte P.L., De Sutter J., Heidbuchel H., Guns P.-J. Toll-like Receptors: Are They Taking a Toll on the Heart in Viral Myocarditis? Viruses. 2021;13:1003. doi: 10.3390/v13061003
[25] Parra-Lucares, Alfredo et al. “Cardiomyopathy Associated with Anti-SARS-CoV-2 Vaccination: What Do We Know?” Viruses vol. 13,12 2493. 13 Dec. 2021, doi:10.3390/v13122493
[26] Morais, Pedro et al. “The Critical Contribution of Pseudouridine to mRNA COVID-19 Vaccines.” Frontiers in cell and developmental biology vol. 9 789427. 4 Nov. 2021, doi:10.3389/fcell.2021.789427
[27] Kranz, L., Diken, M., Haas, H. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016). https://doi.org/10.1038/nature18300
[28] Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005 Aug;23(2):165-75. doi: 10.1016/j.immuni.2005.06.008. PMID: 16111635.
[29] Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005;23:165– 175.
[30] Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 2004;303:1526–1529.
[31] Sameer, Aga Syed, and Saniya Nissar. “Toll-Like Receptors (TLRs): Structure, Functions, Signaling, and Role of Their Polymorphisms in Colorectal Cancer Susceptibility.” BioMed research international vol. 2021 1157023. 12 Sep. 2021, doi:10.1155/2021/1157023
[32] Khanmohammadi, Shaghayegh, and Nima Rezaei. “Role of Toll-like receptors in the pathogenesis of COVID-19.” Journal of medical virology vol. 93,5 (2021): 2735-2739. doi:10.1002/jmv.26826
[33] Williams, Thomas C, and Wendy A Burgers. “SARS-CoV-2 evolution and vaccines: cause for concern?.” The Lancet. Respiratory medicine vol. 9,4 (2021): 333-335. doi:10.1016/S2213-2600(21)00075-8
[34] Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Amir O, Freedman L, Alroy-Preis S, Ash N, Huppert A, Milo R. Protection by a Fourth Dose of BNT162b2 against Omicron in Israel. N Engl J Med. 2022 May 5;386(18):1712-1720.
[35] Mateus J, Grifoni A, Tarke A. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370:89–94.
[36] Moss, P. The T cell immune response against SARS-CoV-2. Nat Immunol 23, 186–193 (2022). https://doi.org/10.1038/s41590-021-01122-w
[37] Angelopoulou A, Alexandris N, Konstantinou E, Mesiakaris K, Zanidis C, Farsalinos K, Poulas K. Imiquimod – A toll like receptor 7 agonist – Is an ideal option for management of COVID 19. Environ Res. 2020;188:109858.
[38] Forthal D. Adaptive immune responses to SARS-CoV-2. Adv Drug Deliv Rev. 2021;172:1-8. doi:10.1016/j.addr.2021.02.009
[39] Sekine, T., Perez-Potti, A., Rivera-Ballesteros, O., Strålin, K., Gorin, J. B., Olsson, A., Llewellyn-Lacey, S., Kamal, H., Bogdanovic, G., Muschiol, S., Wullimann, D. J., Kammann, T., Emgård, J., Parrot, T., Folkesson, E., Karolinska COVID-19 Study Group, Rooyackers, O., Eriksson, L. I., Henter, J. I., Sönnerborg, A., … Buggert, M. (2020). Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell, 183(1), 158–168.e14. https://doi.org/10.1016/j.cell.2020.08.017
[40] Summary of the WHO Expert Meeting on Evaluation of Traditional Chinese Medicine in the Treatment of COVID-19, Feb 28th to 2 Mar 2nd, 2022