Many plant molecules, such as polyphenols, interact with and modulate key regulators of mammalian physiology in ways that are beneficial to health. The more we understand about this interaction, the more effectively we can target both the prevention and treatment of disease.
Polyphenol compounds, when ingested, interact with receptors and enzymes within the consumer. The fact that stress-induced plant compounds tend to upregulate pathways that provide stress resistance in humans and animals suggests that plant consumers may have mechanisms to perceive these chemical cues and react to them in ways that are beneficial. The term xenohormesis is used to explain this phenomenon (from xenos, the Greek word for stranger, and hormesis, the term for health benefits provided by mild biological stress, such as cellular damage or a lack of nutrition).[1]
The objective of Mederi Care/ETMS approach, is to improve patient outcomes by supporting a patient’s autoregulatory capacity utilizing the Mederi Care toolboxes, specifically botanical and nutritional medicine which is applied in a gentle synergistic way. Mederi Care embraces the complexity of diseases by supporting the general idea of autoregulation and addressing underlying dysregulating biological networks. Botanical and nutritional medicine practiced within Mederi Care is primarily directed at enhancement of ‘Self-regulating Internal Community Networks’ supporting and even directing while allowing the freedom to improvise.
The Miracle of Xenohormesis
Xenohormesis is defined as an adaptive response in the physiology of an organism to molecular cues that are neither nutritive nor direct stressors.[2] The xenohormesis hypothesis proposes that xenohormetins are signals of environmental change and trigger a beneficial adaptive response in individuals who consume them.[3]
It is a miracle to think that plants develop important stress protective molecules to defend themselves, and that we can benefit from the protective responses of these plants when we ingest them. These botanical gifts provide us with a gateway to our Creator and is one of the many reasons why I love plant medicine.
Polyphenols such as resveratrol, which is produced by stressed plants, activate sirtuin enzymes and extend the lifespan of fungi and animals, ostensibly by mimicking the beneficial effects of caloric restriction.
Resveratrol belongs to the phytoalexin family of phytochemicals, which are antimicrobial and antioxidant compounds produced by plants in response to fungal infection or physiologic stress.[4] Resveratrol modulates inflammation response in a pleiotropic manner and scavenges free radicals such as superoxide, and may interfere with infections by altering protean cellular pathways.[5] Resveratrol has even been suggested as a compound with immunological benefits that may be useful against Covid-19.[6],[7],[8],[9]
Resveratrol is a stilbene compound, which has been found in a variety of foods as well as in medicinal plants. It is synthesized in various plants—grapes, wines, soy, nuts, and chocolate as a response to pathogenic and stressful environmental situations.[10] The accumulation of resveratrol and other stilbenes in plant cells increases in response to fungal infections (biotic stress) or other physical stresses such as UV radiation, ultrasound, wounds, and invasion of chemicals such as hydrogen peroxide, paraquat etc.[11]
Privileged Structures
Stilbene compounds are part of a vast group of natural defense polyphenols occurring in many plant species. The name “privileged structures” was originally suggested for benzodiazepines but is now generally applied for structures of the class of compounds that bind to several protein-receptor surfaces. More often than not, plants biosynthesize their secondary metabolites for specific reasons. Stilbene compounds can bind several classes of protein structures to elicit a variety of responses.[12]
Resveratrol is a trans-stilbene that undergoes isomerization under UV radiation. Various pharmacological activities have been associated with this compound, and its close analogs have been the subject of numerous studies.[13] Studies show that the beneficial effect of resveratrol on yeast cells is not due to a general property such as antioxidant capacity or nutritive value, but rather a very specific manipulation of a conserved signaling pathway.[14]
Stilbenes enhancing ‘Self-regulating Internal Community Networks’ supporting and even directing while allowing the freedom to improvise.
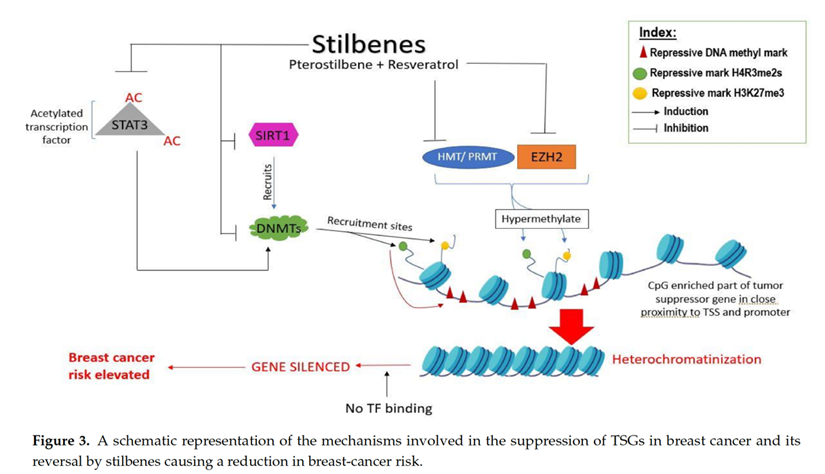
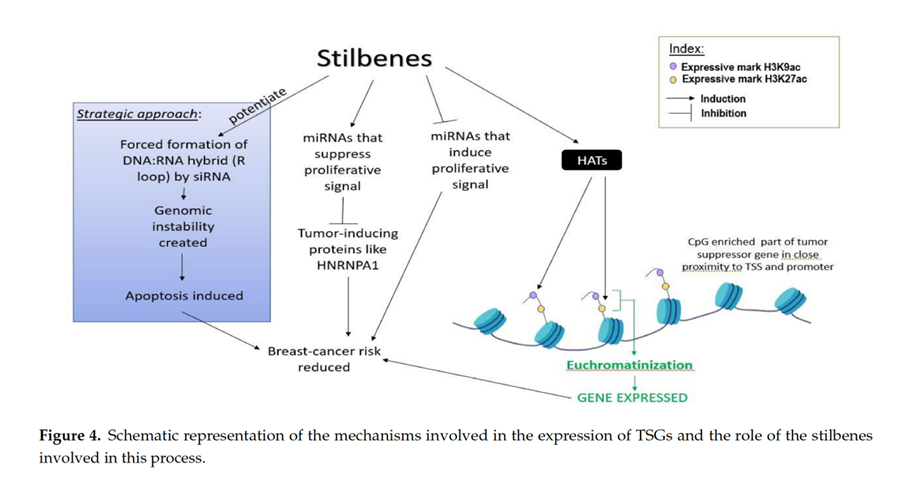
Epigenetics encompasses all the heritable, reversible changes in gene expression, without the genetic code becoming altered. Stilbenes have shown a unique ability to remodel the DNA methylation patterns in breast cancer cells and inhibit oncogenic NOTCH signaling through epigenetic regulation.[15]
Both pterostilbene and resveratrol, in both their natural form and demethylated condition, are capable of upregulating Argonaute2 protein and thereby increased the expression of tumor-suppressive microRNAs like miR-16, miR-141, miR-143, and miR-200c in the triple negative breast cancer cell line MDA-MB-231.[17]
Recent work on breast cancer-associated tumor-suppressive miRNAs (miR-34a, miR-424, and miR-503) demonstrated that these microRNAs are upregulated by resveratrol, which in turn suppresses tumor-inducing protein HNRNPA1 (heterogeneous nuclear ribonucleoprotein A1).[18] HNRNPA1 are a family of conserved nuclear proteins that play key roles in mRNA metabolism, DNA-related functions, and microRNA biogenesis.[19]
Resveratrol as an Emerging Therapeutic Strategy for Cancer
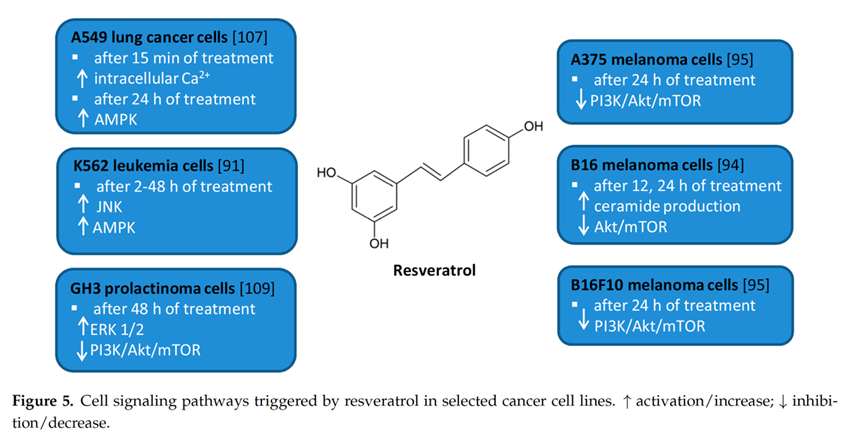
Resveratrol has been proven in numerous studies to have anticancer and chemoprotective effects, as shown in the illustration below.
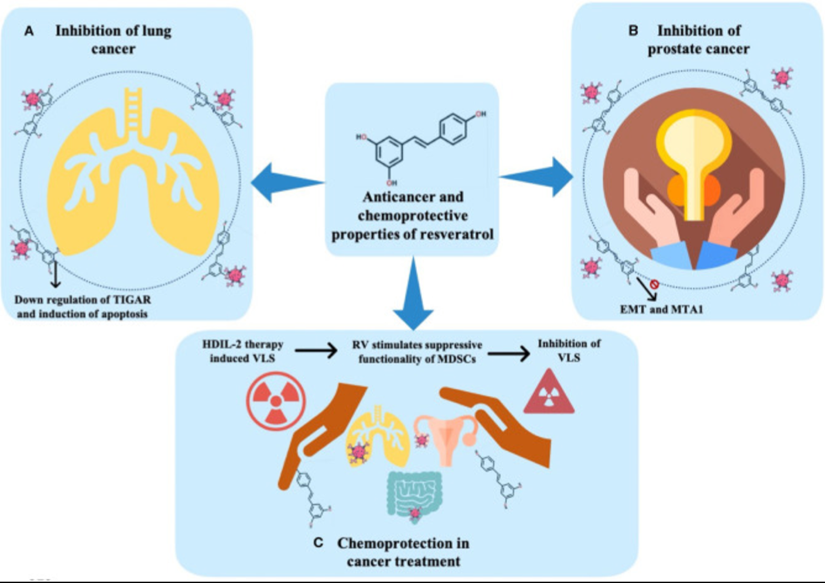
(A) Inhibition of lung cancer. (B) Inhibition of prostate cancer. (C) Chemoprotection in cancer treatment. TIGAR, tumor protein (TP) 53-induced glycolysis and apoptosis regulator; EMT, epithelial-mesenchymal transition; MTA, metastasis-associated protein; HDIL-2, high-dose interleukin-2; RV, resveratrol; MDSCs, myeloid-derived suppressor cells; VLS, vascular leak syndrome.[20]
Resveratrol exerts positive benefits in numerous ways. It tends to induce apoptosis and has been used in carcinopreventive activity studies. Resveratrol has also been shown to possess significant inhibitory activities against different kinds of malignant primary tumors, including breast cancer, colon cancer, and prostate cancer.[21],[22],[23]
Studies indicate that resveratrol modulates apoptosis and autophagy as the main forms of cancer cell deaths by targeting various signaling pathways and up/downregulation of apoptotic and autophagic genes.[24] Resveratrol has been shown to modulate autophagy in many types of cancer including leukemia, melanoma, glioma as well as renal, esophageal, liver, colon, prostate, breast, ovarian, oral, and lung cancer.[25],[26]
Furthermore, resveratrol is able to suppress cancer stems cells (CSCs) features by reducing Wnt/β-catenin in breast cancer.[27] Resveratrol triggers autophagy in CSCs and reduces breast cancer progression. It is important to note the resveratrol promotes auto-regulation of autophagy.[28],[29] Autophagy has both tumor-promoting and tumor-suppressor roles in cancer.
Recent clinical trials using low or moderate doses of resveratrol have shown a positive effect on metabolic parameters and oxidative stress in many chronic diseases as well as cancer.[31],[32]
Promising Research on Resveratrol as a Cancer Preventative
Resveratrol has received attention as a cancer preventative and antagonist of the aromatic hydrocarbon receptor (AHR).[33] In cell culture experiments with MCF-10A cells, resveratrol (1 to 5 µM) protected against chemical (polyaromatic hydrocarbons)-induced DNA damage by suppressing expression of CYP1A1 and CYP1B1.[34]
In studies with Sprague-Dawley rats, dietary supplementation starting at birth and with serum levels approaching 2.0 µM suppressed DMBA induced mammary cancer.[35] The protective effects of resveratrol against DMBA induced tumorigenesis were also seen when the supplementation started later in life (45 days of age).[36]
In MCF-7 cells, at doses (1.0 µM) that approximated those achieved (2.4 µM) in human serum, resveratrol antagonized AHR-dependent repression of BRCA-1 transcription; deacetylation of H4 associated with BRCA-1; and BRCA-1 CpG hypermethylation while reducing BRCA-1 association of AHR, MBD-2, and DNMT-1.[37] In mammary tissue of Sprague-Dawley rat female offspring, researchers found that maternal supplementation with resveratrol antagonized AHR-mediated downregulation of BRCA-1 and Brca-1 hypermethylation.[38]
Resveratrol appears to maintain cellular allostasis in part by protecting cells against oxidative injury and other cancer-causing perturbations. The addition of resveratrol to standard chemotherapeutic regimens appears to be helpful in preventing the development of secondary malignancies that result from mutagenic effects of chemotherapy and radiotherapy.[39],[40],[41],[42]
Resveratrol Enhances Chemotherapy and Protects Against Chemotherapy Damage
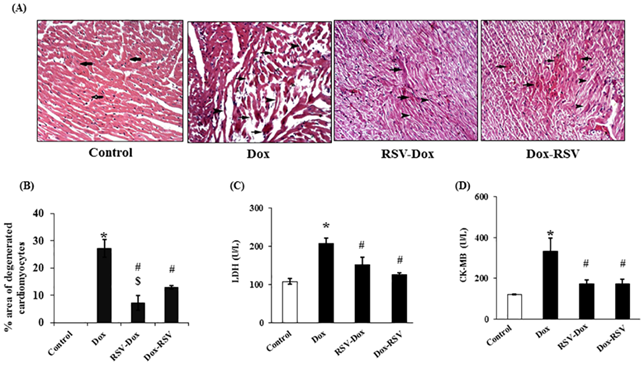
Resveratrol may also help to prevent other long-term morbidities associated with anti-cancer therapy, such as cardiac myocyte toxicity and subsequent heart failure from exposure to anthracyclines such as doxorubicin (DOX).[43],[44]
Both prophylactic and therapeutic use of resveratrol mitigates DOX induced deterioration of cardiac function as assessed by echocardiography. Also, resveratrol treatment (prophylactic and therapeutic) prevented DOX-induced myocardial damage as measured by cardiac enzymes (LDH and CK-MB) in serum. This was associated with decrease in DOX induced myocardial apoptosis and fibrosis. This study reveals that prophylactic use of RSV was more effective than its therapeutic use in mitigating DOX induced apoptosis and fibrosis in the myocardium. Therefore, prophylactic use of resveratrol should be considered as a possible future adjuvant therapy to minimize cardiotoxic side effects of doxorubicin in cancer patients.
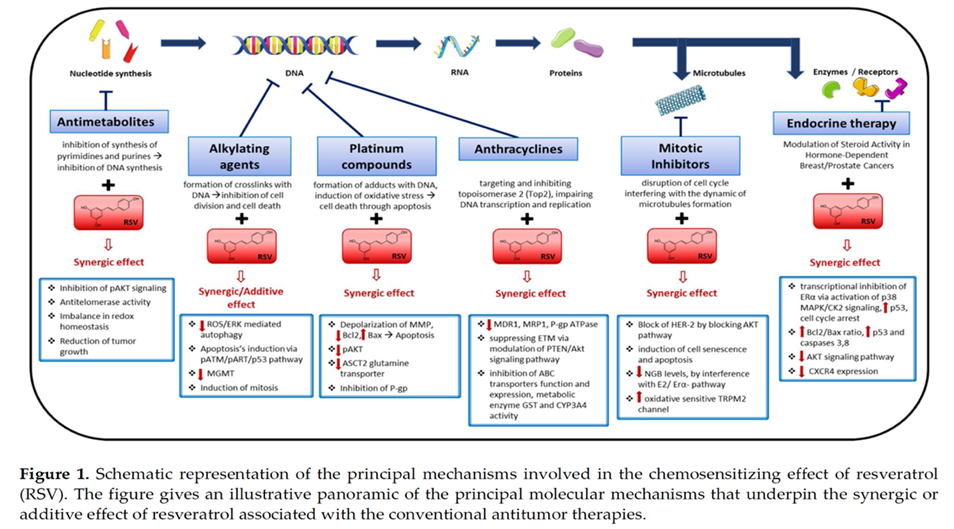
Resveratrol can sensitize tumor cells to chemotherapeutic agents. The tumors shown to be sensitized by resveratrol include lung carcinoma, acute myeloid leukemia, promyelocytic leukemia, multiple myeloma, prostate cancer, oral epidermoid carcinoma, and pancreatic cancer. The chemotherapeutic agents include vincristine, adriamycin, paclitaxel, doxorubicin, cisplatin, gefitinib, 5-fluorouracil, velcade, and gemcitabine.
The chemosensitization of tumor cells by resveratrol appears to be mediated through its ability to modulate multiple cell-signaling molecules, including drug transporters, cell survival proteins, cell proliferative proteins, and members of the NF-κB and STAT3 signaling pathways. Overall, studies suggest that resveratrol can be used to sensitize tumors to standard cancer chemotherapeutics.[46]
Mechanism of chemosensitization of tumors by resveratrol. Resveratrol sensitizes tumor cells to chemotherapeutic agents by targeting proteins involved in cell survival, cell proliferation, and drug transport. ↓, down-regulation, ↑, up-regulation, ↑ activation.
Mechanism of chemosensitization of tumors by resveratrol. Resveratrol sensitizes tumor cells to chemotherapeutic agents by targeting proteins involved in cell survival, cell proliferation, and drug transport. ↓, down-regulation, ↑, up-regulation, ↑ activation.
Resveratrol appears as a promising molecule able to sensitize resistant tumors to drugs, suggesting its potential use in therapy-refractory cancer patients.
Combinatorial Treatment with Ursolic acid, Curcumin, and Resveratrol Inhibits Prostate Tumor Growth and Leads to Key Modulations of Cancer Cell Metabolism
Within the wholistic medical system we call Mederi Care, bathing the body with a full orchestra of plant compounds is common. We rarely, if ever, use single plants. Our philosophy is to combine the synergy of various plants working together in harmony. A good example is combining ursolic acid from sage or basil, curcumin from turmeric, and resveratrol.
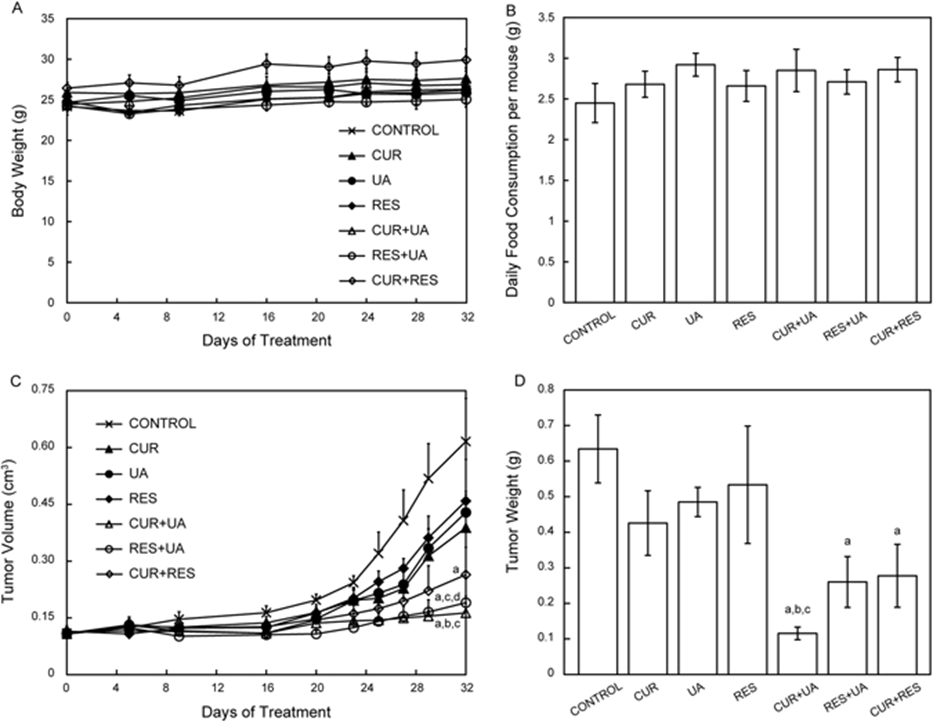
In a research study, ursolic acid, curcumin and resveratrol were selected for further analyses and administered in vivo via the diet, either alone or in combination, in a mouse allograft model of prostate cancer. All possible combinations of these natural compounds produced synergistic effects on tumor size and weight, as predicted in the screens. A subsequent untargeted metabolomics and metabolic flux analysis using isotopically labeled glutamine indicated that the compound combinations modulated glutamine metabolism. In addition, ASCT2 levels and STAT3, mTORC1 and AMPK activity were modulated to a greater extent by the combinations compared to the individual compounds. Overall, this approach can be useful for identifying synergistic combinations of natural compounds for chemopreventive and therapeutic interventions.
In vivo effect on HMVP2 tumor growth of treatment with CUR, UA, RES and their combinations. HMVP2 cell spheroids were injected subcutaneously into the flank of male FVB/N mice. Mice were fed ad libitum with semi-purified AIN76A-based diet containing CUR, UA, RES or their combinations of two natural compounds. Body weight (a), food consumption (b), tumor volume (c) and tumor weight (d) are shown as mean ± SEM. One-way ANOVA with significance at p < 0.05 was used. Statistical significance is shown as different from control (a), CUR (b), UA (c) and RES (d)[48]
It is evident from such studies that resveratrol presents a myriad of beneficial effects on health and acts at multiple levels including cellular signaling, enzymatic pathways, apoptosis, and gene expression to promote and optimize health. It’s possible that resveratrol may be useful for preventing and treating disease as well.[49]
Based on both clinical experience and the scientific literature, resveratrol is most effective when combined with several other “privileged structures” such as curcumin from turmeric, catechins from green tea, other flavonoids from grapeseed, diterpenes from rosemary, thymoquinone from black cumin seed, and gingerols from ginger. It appears that there is great synergy and harmony between these various compounds. From a standpoint of health optimization, I believe that using botanicals together, in carefully formulated combinations, is the most effective approach for supporting health.
References
[1] Howitz KT, Sinclair DA. Xenohormesis: sensing the chemical cues of other species. Cell. 2008 May 2;133(3):387-91.
[2] Baur JA, Sinclair DA. What is Xenohormesis? Am J Pharmacol Toxicol. 2008 Mar 31;3(1):152-159.
[3] de Medina P. Xenohormesis in early life: New avenues of research to explore anti-aging strategies through the maternal diet. Med Hypotheses. 2017 Nov;109:126-130. doi: 10.1016/j.mehy.2017.10.005. Epub 2017 Oct 12. PMID: 29150271.
[4] Campagna M, Rivas C. Antiviral activity of resveratrol. Biochem Soc Trans 2010;38:50-3.
[5] Abba Y, Hassim H, Hamzah H, Noordin MM. Antiviral activity of resveratrol against human and animal viruses. Adv Virol 2015;184241.doi:10.1155/2015/184241.
[6] Kunnumakkara, Ajaikumar B et al. “COVID-19, cytokines, inflammation, and spices: How are they related?.” Life sciences vol. 284 (2021): 119201. doi:10.1016/j.lfs.2021.119201
[7] Pasquereau, Sébastien et al. “Resveratrol Inhibits HCoV-229E and SARS-CoV-2 Coronavirus Replication In Vitro.” Viruses vol. 13,2 354. 23 Feb. 2021, doi:10.3390/v13020354
[8] Ellen ter B.M., Dinesh Kumar N., Bouma E.M., Troost B., Pol van de D.P.I., Ende van derMetselaar H.H., Apperloo L., Gosliga van D., Berge van den M., Nawijn M.C., Voort van der P.H.J., Moser J., Rodenhuis-Zybert I.A., Smit J.M. Resveratrol and pterostilbene potently inhibit SARS-COV-2 infection in vitro. bioRxiv. 2020 doi: 10.1101/2020.09.24.285940.
[9] Marinella, Mark A. “Indomethacin and resveratrol as potential treatment adjuncts for SARS-CoV-2/COVID-19.” International journal of clinical practice vol. 74,9 (2020): e13535. doi:10.1111/ijcp.13535
[10] Antus, C.; Radnai, B.; Dombovari, P.; Fonai, F.; Avar, P.; Matyus, P.; Racz, B.; Sumegi, B.; Veres, B. Anti-inflammatory effects of a triple-bond resveratrol analog: Structure and function relationship. Eur. J. Pharmacol. 2015, 748, 61–67.
[11] Ibern-Gómez M, Roig-Pérez S, Lamuela-Raventós RM, de la Torre-Boronat MC. Resveratrol and piceid levels in natural and blended peanut butters. J Agric Food Chem. 2000 Dec;48(12):6352-4. doi: 10.1021/jf000786k. PMID: 11312807.
[12] J. Chong, A. Poutaraud, and P. Hugueney, “Metabolism and roles of stilbenes in plants,” Plant Science, vol. 177, no. 3, pp. 143– 155, 2009. http://www.sabinsa.com/newsroom/articles-download/932-sabinsa-article-3
[13] Rimando AM, Suh N. Biological/chemopreventive activity of stilbenes and their effect on colon cancer. Planta Med. 2008 Oct;74(13):1635-43.
[14] Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003 Sep 11; 425(6954):191-6.
[15] Lubecka K, Kurzava L, Flower K, Buvala H, Zhang H, Teegarden D, Camarillo I, Suderman M, Kuang S, Andrisani O, Flanagan JM, Stefanska B. Stilbenoids remodel the DNA methylation patterns in breast cancer cells and inhibit oncogenic NOTCH signaling through epigenetic regulation of MAML2 transcriptional activity. Carcinogenesis. 2016 Jul;37(7):656-68. doi: 10.1093/carcin/bgw048. Epub 2016 Apr 28.
[16] Ganguly S, Arora I, Tollefsbol TO. Impact of Stilbenes as Epigenetic Modulators of Breast Cancer Risk and Associated Biomarkers. Int J Mol Sci. 2021 Sep 17;22(18):10033. doi: 10.3390/ijms221810033.
[17] Hagiwara, K.; Kosaka, N.; Yoshioka, Y.; Takahashi, R.-u.; Takeshita, F.; Ochiya, T. Stilbene derivatives promote Ago2-dependent tumour-suppressive microRNA activity. Sci. Rep. 2012, 2, 314.
[18] Otsuka, K.; Yamamoto, Y.; Ochiya, T. Regulatory role of resveratrol, a microRNA-controlling compound, in HNRNPA1 expression, which is associated with poor prognosis in breast cancer. Oncotarget 2018, 9, 24718–24730.
[19] Bekenstein U, Soreq H. Heterogeneous nuclear ribonucleoprotein A1 in health and neurodegenerative disease: from structural insights to post-transcriptional regulatory roles. Mol Cell Neurosci. 2013 Sep;56:436-46. doi: 10.1016/j.mcn.2012.12.002. Epub 2012 Dec 14. PMID: 23247072.
[20] Sharifi-Rad J, Quispe C, Mukazhanova Z, Knut E, Turgumbayeva A, Kipchakbayeva A, Seitimova G, Mahomoodally MF, Lobine D, Koay A, Wang J, Sheridan H, Leyva-Gómez G, Prado-Audelo MLD, Cortes H, Rescigno A, Zucca P, Sytar O, Imran M, Rodrigues CF, Cruz-Martins N, Ekiert H, Kumar M, Abdull Razis AF, Sunusi U, Kamal RM, Szopa A. Resveratrol-Based Nanoformulations as an Emerging Therapeutic Strategy for Cancer. Front Mol Biosci. 2021 Sep 1;8:649395. doi: 10.3389/fmolb.2021.649395.
[21] Patrick WM, Juile AA, John GS et al: Pterostilbene inhibits pancreatic cancer in vitro. J Gastrointest Surg, 2010; 14(5): 873–79
[22] Rimando AM, Suh N: Biological/chemopreventive activity of stilbenes and their effect of colon cancer. Planta Med, 2008; 74(13): 1635–43
[23] Blanquer-Rossell. MD, Hern.ndez-L.pez R, Roca P et al: Resveratrol induces mitochondrial respiration and apoptosis in SW620 colon cancer cells. Biochim Biophys Acta, 2016. Sun Y, Wu X, Cai X et al: Identification of pinostilbene as a major colonic metabolite of pterostilbene and its inhibitory effects on colon cancer cells. Mol Nutr Food Res, 2016; 60(9): 1924–32
[24] Ashrafizadeh M, Taeb S, Haghi-Aminjan H, Afrashi S, Moloudi K, Musa AE, Najafi M, Farhood B. Resveratrol as an Enhancer of Apoptosis in Cancer: A Mechanistic Review. Anticancer Agents Med Chem. 2021;21(17):2327-2336. doi: 10.2174/1871520620666201020160348. PMID: 33081687.
[25] Yang R, Dong H, Jia S, Yang Z. Resveratrol as a modulatory of apoptosis and autophagy in cancer therapy. Clin Transl Oncol. 2022 Jan 17. doi: 10.1007/s12094-021-02770-y.
[26] Fu X, Li M, Tang C, Huang Z, Najafi M. Targeting of cancer cell death mechanisms by resveratrol: a review. Apoptosis. 2021 Dec;26(11-12):561-573. doi: 10.1007/s10495-021-01689-7. Epub 2021 Sep 25. PMID: 34561763.
[27] Fu, Y.; Chang, H.; Peng, X.; Bai, Q.; Yi, L.; Zhou, Y.; Zhu, J.; Mi, M. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/β-catenin signaling pathway. PLoS ONE 2014, 9, e102535.
[28] Ashrafizadeh, M.; Ahmadi, Z.; Farkhondeh, T.; Samarghandian, S. Modulatory effects of statins on the autophagy: A therapeutic perspective. J. Cell. Physiol. 2020, 235, 3157–3168
[29] Ashrafizadeh, M.; Zarrabi, A.; Orouei, S.; Hushmandi, K.; Hakimi, A.; Zabolian, A.; Daneshi, S.; Samarghandian, S.; Baradaran, B.; Najafi, M. MicroRNA-mediated autophagy regulation in cancer therapy: The role in chemoresistance/chemosensitivity. Eur. J. Pharmacol. 2020, 892, 173660.
[30] Musial, K, Siedlecka-Kroplewska, K , Kmiec, Z. Gorska-Ponikowska, M., Modulation of Autophagy in Cancer Cells by Dietary Polyphenols, Antioxidants 2021, 10, 123. https://doi.org/10.3390/antiox10010123
[31] Leon, D.; Uribe, E.; Zambrano, A.; Salas, M. Implications of Resveratrol on Glucose Uptake and Metabolism. Molecules 2017, 22
[32] Carter, Lindsay G et al. “Resveratrol and cancer: focus on in vivo evidence.” Endocrine-related cancer vol. 21,3 R209-25. 6 May. 2014, doi:10.1530/ERC-13-0171
[33] Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003; 43:309–334.
[34] Leung HY, Yung LH, Shi G, Lu AL, Leung LK. The red wine polyphenol resveratrol reduces polycyclic aromatic hydrocarbon-induced DNA damage in MCF-10A cells. Br J Nutr. 2009; 102:1462–8.
[35] Whitsett TG Jr, Lamartiniere CA. Genistein and resveratrol: mammary cancer chemoprevention and mechanisms of action in the rat. Expert Rev Anticancer Ther. 2006; 6:1699–706.
[36] Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthraceneinduced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002; 62:4945–54.
[37] Papoutsis AJ, Lamore SD, Wondrak GT, Selmin OI, Romagnolo DF. Resveratrol prevents epigenetic silencing of BRCA-1 by the aromatic hydrocarbon receptor in human breast cancer cells. J. Nutr. 2010; 140:1607–14.
[38] Papoutsis AJ, Borg JL, Selmin OI, Romagnolo DF. BRCA-1 promoter hypermethylation and silencing induced by the aromatic hydrocarbon receptor-ligand TCDD are prevented by resveratrol in MCF-7 cells. J. Nutr. Biochem. 2012; 23:1324–32.
[39] Kinghorn AD, Su BN, Jang DS, Chang LC, Lee D, Gu JQ, Carcache-Blanco EJ, Pawlus AD, Lee SK, Park EJ, Cuendet M, Gills JJ, Bhat K, Park HS, Mata-Greenwood E, Song LL, Jang M, Pezzuto JM. Natural inhibitors of carcinogenesis. Planta Med. 2004 Aug;70(8):691-705. doi: 10.1055/s-2004-827198. PMID: 15326546.
[40] Aziz MH, Reagan-Shaw S, Wu J, Longley BJ, Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease? FASEB J. 2005 Jul;19(9):1193-5. doi: 10.1096/fj.04-3582fje. Epub 2005 Apr 18. Erratum in: FASEB J. 2005 Jul;19(9):1 p following 1195. PMID: 15837718.
[41] Kundu JK, Surh YJ. Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Letters. 2008;269:243–261.
[42] Dennis T, Fanous M, Mousa S. Natural products for chemopreventive and adjunctive therapy in oncologic disease. Nutr Cancer. 2009;61(5):587-97. doi: 10.1080/01635580902825530. PMID: 19838932.
[43] Tatlidede E, Sehirli O, Velioglu-Ogunc A, Cetinel S, Yegen BC, Yarat A, Suleymanoglu S, Sener G. Resveratrol treatment protects against doxorubicin-induced cardiotoxicity by alleviating oxidative damage. Free Radical Research. 2009;43:195–205. doi: 10.1080/10715760802673008.
[44] Hu LF, Lan HR, Li XM, Jin KT. A Systematic Review of the Potential Chemoprotective Effects of Resveratrol on Doxorubicin-Induced Cardiotoxicity: Focus on the Antioxidant, Antiapoptotic, and Anti-Inflammatory Activities. Oxid Med Cell Longev. 2021 Aug 22;2021:2951697. doi: 10.1155/2021/2951697. PMID: 34471463; PMCID: PMC8405305.
[45] Shoukry HS, Ammar HI, Rashed LA, Zikri MB, Shamaa AA, Abou Elfadl SG, Rub EA, Saravanan S, Dhingra S. Prophylactic supplementation of resveratrol is more effective than its therapeutic use against doxorubicin induced cardiotoxicity. PLoS One. 2017 Jul 20;12(7):e0181535. doi: 10.1371/journal.pone.0181535. eCollection 2017.
[46] Gupta SC, Kannappan R, Reuter S, Kim JH, Aggarwal BB. Ann N Y Acad Sci. 2011 Jan;1215:150-60. doi: 10.1111/j.1749-6632.2010.05852.x. Review. Chemosensitization of tumors by resveratrol.
[47] Cocetta V, Quagliariello V, Fiorica F, Berretta M, Montopoli M. Resveratrol as Chemosensitizer Agent: State of Art and Future Perspectives. Int J Mol Sci. 2021 Feb 19;22(4):2049. doi: 10.3390/ijms22042049. PMID: 33669559;
[48] Lodi A, Saha A, Lu X, Wang B, Sentandreu E, Collins M, Kolonin MG, DiGiovanni J, Tiziani S. Combinatorial treatment with natural compounds in prostate cancer inhibits prostate tumor growth and leads to key modulations of cancer cell metabolism. NPJ Precis Oncol. 2017;1. pii: 18. doi: 10.1038/s41698-017-0024-z.
[49] Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug. Discov. 2006, 5, 493–506.