Commentary on the Widely Disseminated Article entitled “Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients with Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG SO221)” Published in the Journal of Clinical Oncology, December 2019
The DELCaP study, recently published in the Journal of Clinical Oncology evaluating the use of dietary supplements during chemotherapy, has alarmed many patients and raised troubling questions for health practitioners.
The purpose of the study was to evaluate associations between ‘antioxidant’ supplement use and breast cancer outcomes in light of the widespread use of supplements during cancer therapies and the ongoing debate over concerns that antioxidants could reduce the cytotoxic effects of reactive oxygen species (ROS) generated by chemotherapy agents. The authors claim that the use of dietary supplements before and during chemotherapy is associated with an increased risk of recurrence and, to a lesser extent, death.1
Understandably, their conclusion is distressing for patients and concerning for health practitioners.
A recent review of the study published in the Natural Medicine Journal stated: “The study’s main conclusion, which suggests that the use of all antioxidants during chemotherapy may be harmful, suffers from numerous limitations and should be reframed and revised.” 2
Concerns About Cancer and Antioxidants: Much Ado About Nothing?
I spend a great deal of time reading and evaluating clinical studies, particularly those related to cancer. Of course, any research on the relationship of botanicals and nutrients to cancer is especially pertinent to my work, and therefore to the Mederi Care approach practiced at our clinic. Our practice paradigm in our clinic is continually evolving and staying abreast of the most current research is of utmost importance.
Questions related to the effects of antioxidants on cancer arise from time to time. In fact, in 2014 I addressed this issue in a post entitled, “Do Antioxidants Prevent or Accelerate Cancer?” In light of the current study that is once again raising concerns, it is important to understand that observational research, such as in this study, identifies associations within a population, where the investigators are not directly controlling variables (compared to clinical trials where there is specific control over what participants are exposed to).2
Simply put, the attention this article has attracted is ‘much ado about nothing’ and caution should be taken not to suggest it constitutes evidence that can be used as the basis for clinical care decisions, such as avoiding supplementation, or weighed heavily in the absence of more robust research. Importantly, the type of supplementation described in this article is not reflective of a chemotherapy-supportive supplementation program designed using the Mederi Care approach practiced at the Mederi Center. Within the Mederi Care approach, we use only specific forms of natural compounds, in balanced and personalized formulas that are adapted to the individual’s specific cancer and conventional treatment type, current health status and internal microenvironment (based on laboratory testing). Breast cancer patients should seek the guidance of an expert in botanical and nutritional medicine regarding which supplements to take, in what form, and how often before, during, and after their course of treatment.
It is also important to note that ‘dietary supplements’ is a broad term within which there are various categories that cannot all be termed ‘antioxidants’. Specific antioxidants included in this study were: multivitamins, vitamin A, vitamin B12, vitamin C, vitamin D, vitamin E, coenzyme Q10, carotenoids, folic acid, iron, omega-3 fatty acids (fish oil, EPA, omega-3, flaxseed, or cod liver oil), calcium, glucosomine, melatonin, and acidophilus.
Problems with This Particular Study
For this study, the authors randomly assigned breast cancer patients to different treatment schedules of doxorubicin, cyclophosphamide and paclitaxel or placebo and queried them on their use of supplements before and during chemotherapy treatment. This was an association study, which is considered to be the weakest of all study types.
Findings of the study include the following:
- “In the 1,134 patients who completed both questionnaires, 251 recurrences and 181 deaths occurred. In this group, for the fully adjusted model, use of any antioxidant (grouped) both before and during chemotherapy was non-significantly associated with recurrence (adjusted hazard ratio [adjHR]: 1.41; 95% confidence interval [CI]: 0.98-2.04; P=0.06) and survival (adjHR: 1.40; 95% CI: 0.90-2.18; P=0.14).”
- When analyzed in isolation, use of any antioxidant only before or only during was not associated with worsening of either endpoint. The use of single antioxidants vitamin C, vitamin E, and coenzyme Q10 (CoQ10) did not have statistically significant associations.
- Use of vitamin A both before and during chemotherapy was associated with a significantly increased risk of recurrence (adjHR: 4.23; 95% CI: 1.32-13.57) and death (adjHR: 3.53; 95% CI: 1.05-11.93), but not carotenoids (significant increase in recurrence for use only during).
- Reported use of multivitamins and vitamin D either before or during chemotherapy or for both time points was not associated with survival outcomes.”2
“It is of note that only a small portion of the total patient population used supplements. For example, only 138 patients (12.2%) reported using oral vitamin C during chemotherapy. It is important to keep in mind the fairly small sample size from which the author’s conclusions stem, ranging between 5 to 551 people assessed based on use of each supplement (this is a limitation aptly presented by the study authors).”2
Additionally, the study found no significant relationships between antioxidant use and outcomes when the supplements were taken only before treatment initiation or only during chemotherapy. Additionally, associations for the majority of single antioxidant supplements were not statistically significant.
According to the study, while relationships for disease free survival (DFS) were similar by estrogen receptor (ER)+ status, the association with overall survival (OS) was driven largely by ER+ breast cancer with no association for ER- disease. The study itself referred to the finding as borderline for significance. Older woman with ER+ breast cancer rarely receive chemotherapy, unless they have advanced on all other therapies including estrogen antagonists, aromatase inhibitors, CDK4/6 inhibitors, mTOR inhibitors and PIK-3 inhibitors. This means they are most likely in poorer overall health and have more advanced disease (disease stage was not available for this study). This is a red flag.
Further, when conducting a meaningful clinical trial, the recommendations for reporting with combinations of antioxidants and chemotherapy need to reveal the mechanism(s) of action of specific combinations of antioxidants and conventional cancer therapies.
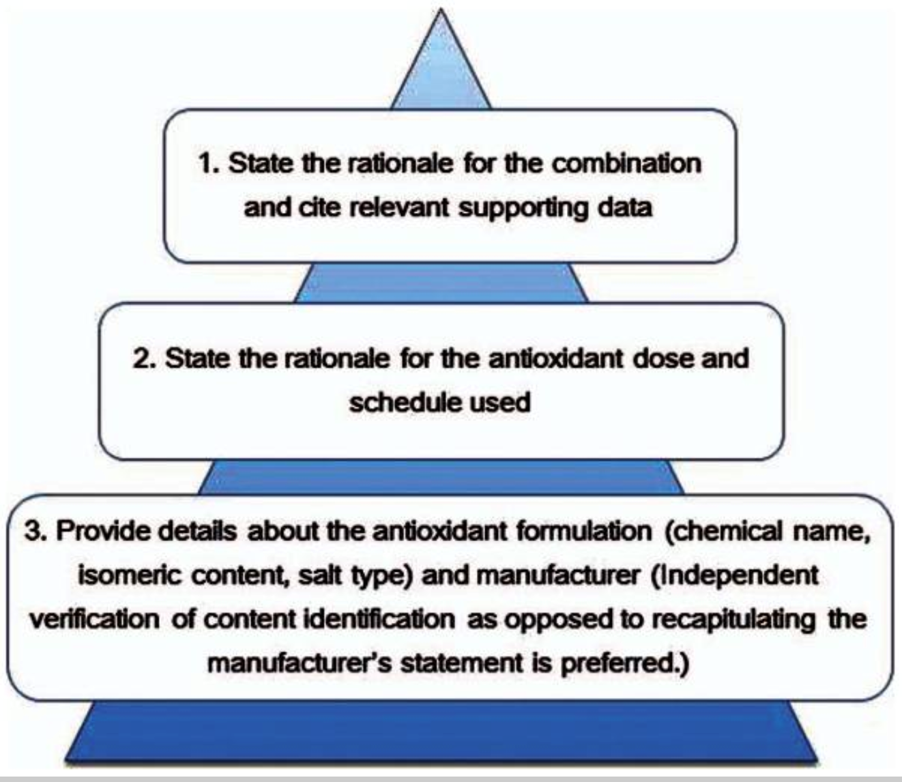
The above recommended guidelines were not followed in this study.
The Hierarchy of Evidence: Is the Study’s Design Robust?
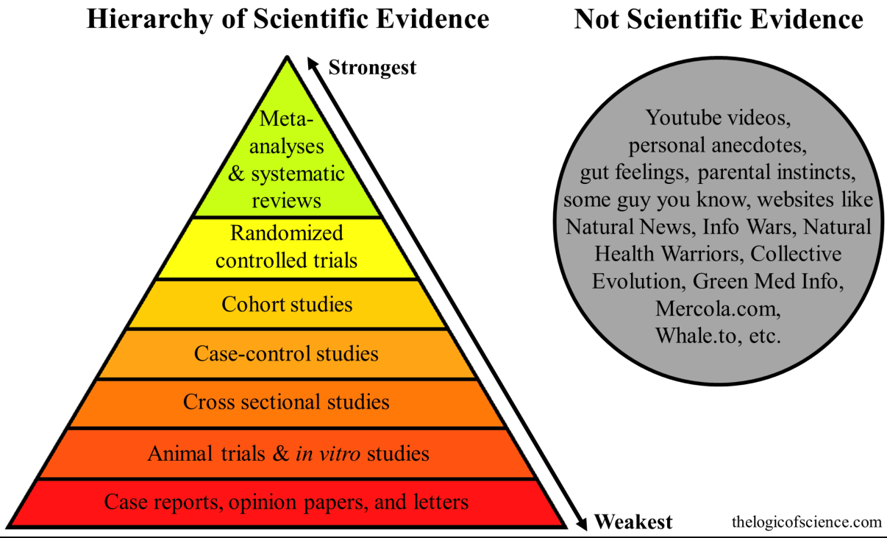
It is a well-known fact that people are extraordinarily prone to confirmation biases. We have a strong tendency to latch onto anything that supports our position and blindly ignore anything that doesn’t.4
Two of the cornerstones of science advancement are rigor in designing and performing scientific research and the ability to reproduce biomedical research findings. The application of rigor ensures robust and unbiased experimental design, methodology, analysis, interpretation, and reporting of results.
There are five main types of bias and methods of bias control:
- Selective bias – Randomized treatment assignment and concealment of assessment.
- Bias in study management – Standardized operating procedures and training of research personnel.
- Ascertainment bias – Blinding of researchers to treatment assignment.
- Bias introduced after randomization – Intention-to-treat analysis.
- Publication bias – Prospective registration of trials and withholding publication of negative trials.
Studies involving questionnaires are always difficult to interpret. While this was a prospective study ancillary to a therapeutic trial, the study states that “as in many observational studies, there was the potential for a number of biases, including selection, recall, and confounding biases”. 1

The premise for this study seems to be that antioxidant use during chemotherapy could reduce the cytotoxic effects of reactive oxygen species generated by numerous chemotherapy agents. However, many different kinds of chemotherapy drugs are used to treat cancer, either alone or in combination with other drugs or treatments. These drugs are very different in their mechanisms of action and chemical compositions, as well as their toxicity profiles.
Most chemotherapy drugs do not actually induce anti-tumor effects by imposing oxidative damage, but some do. Each drug and/or group of drugs works differently to destroy cancer cells, and each has different adverse effects, stresses different organ systems, and has varying long-term toxicity. Therefore, each chemotherapy protocol requires distinct supplements to mitigate adverse effects and toxicity, improve recovery, enhance benefits, and reduce drug resistance.
Because there is so much variation in chemotherapy agents, what does this study really say about antioxidant use and chemotherapy?
Understanding Antioxidants
The word antioxidant is vague and probably should not be applied to nutrients or herbs at all. The authors’ use of the word antioxidant is misleading, as it appears that all dietary supplements used are lumped into this category, regardless of type, form or dosage.
While vitamin C, vitamin A, vitamin E, carotenoids, or coenzyme Q10 could act as antioxidants, it is unclear what forms or dosages were actually used.
This is important because Vitamin C for example, supplemented in its isolated form (ascorbic acid), can have antioxidant or pro-oxidant activity depending on the dose, and environment.30-32 Organic natural vitamin C in food or the Naturized supplemental form will not become pro-oxidative and generate increased oxidative damage, as ascorbic acid can.33
Furthermore, it is unclear why iron, omega-3 fatty acids, and vitamin B12 are included in this study. According to the authors, while an association between iron and vitamin B12 supplementation and diminished progression free survival and overall survival was found, after those two supplements (which aren’t actually antioxidants at all) were controlled for, the relationship was found to be either non-significant or non-robust.
The following are direct quotations from the study discussion that should be considered:
“Patients who took any antioxidant, as well as vitamin B12 and iron, tended to be older than those who did not.”
“A possibility exists that patients who chose to take supplements were less likely to adhere to adjuvant hormonal therapy, which could be associated with poorer survival outcomes, but information on adherence was not available for this trial.”
“It is possible that relationships with the use of antioxidants during chemotherapy could be primarily among patients who also received adjuvant radiation subsequent to this trial.”
“Although a landmark analysis approach appropriately separates predictor (supplement use) from survival outcomes, it does not rule out the possibility of selection bias between groups.”
Example 1: Form and Quality Variation Among Iron Supplements
Differences in form and quality of supplements can also cause confusion when one tries to make comparisons between what appears to be the same supplement. This is as a major oversight of this study.
For example, there are four main types of iron supplements: ferrous sulphate, ferrous gluconate and ferrous fumarate, which are iron isolates, and naturized iron, which isn’t an isolate at all, but a form of iron embedded in a wholefood matrix. In naturized supplements, Saccharomyces cerevisiae or Lactobacillus bulgaricus cultures are grown with the desired nutrient so that it becomes fully integrated into the whole-food matrix, virtually mimicking nature. This creates an easily digested, highly absorbable whole iron complex with high-quality protein that contains essential and non-essential amino acids, vitamins, and minerals.5-13 The bio-yogurt culture also provides many intrinsic nutrients that enhance absorption.11-13
Other benefits of Saccharomyces cerevisiae or Lactobacillus bulgaricus include: stabilization of the rumen pH, stimulation of certain cellulolytic beneficial bacteria, enhanced fiber degradation, reduced methane production and nitrogen loss, and enhancement of the immune system.5-13
Many commercially available iron supplements can act in a pro-oxidant fashion for a variety of reasons.
As mentioned above, the majority of iron supplements on the market are of the iron isolate form. Iron isolates often remain unabsorbed and hence may be available to participate in Fenton-driven free radical generation.14 Standard iron supplementation can actually induce oxidative damage and promote cardiovascular disease15,16 and cancer growth, especially when taken in higher doses in an oxidative environment, such as in a patient receiving chemotherapy and/or radiation therapy.17-20
Recent studies have also shown that iron plays a role in the tumor microenvironment and in metastasis. 18,19 Iron also has the potential to promote cancer cell proliferation, because it contributes to DNA synthesis. Cancer cells often have a high iron requirement for this reason. Mounting evidence indicates that changes in iron metabolism-related proteins contribute to tumor initiation and growth. Additionally, authors of a recent review suggest that iron intake may have different effects on breast cancer risk according to menopausal and hormone receptor status, as well as genotypes affecting antioxidant capacity.20
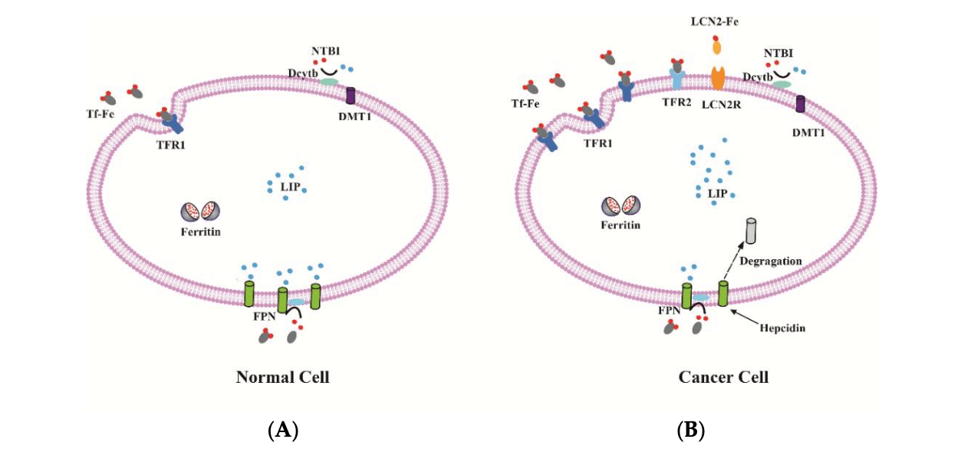
Iron metabolism in normal and cancer cell. (A) The expression of iron uptake proteins, such as TFR1, TFR2 and DMT1 are low, and the expression of iron export protein FPN is high in normal cells, resulting in a low level of labile iron; (B) By contrast, some cancer cells often have elevated TFR1, TFR2 and LCN2 as well as low FPN expression. Therefore, these cancer cells can increase intracellular iron to maintain the high demand for iron.18
An iron-loading phenotype of cancer cells is linked to accelerated tumor progression.19-25
Blanket Recommendations and Self-prescribed Iron Supplementation is Problematic
Anemia is extremely common and occurs in ~40–70% of cancer patients.26,27 Anemia is also a crucial indicator of cancer risk,28 and iron deficiency anemia is the most significant contributor, accounting for 50% of all causes of anemia.24 A systematic literature review of reports from 1966 through 2003 identified the prevalence of anemia in specific cancers and assessed the impact of anemia on survival and quality of life. Patients with anemia had poorer survival and local tumor control than did their non-anemic counterparts in 15 of 18 studies. 29
When a person presents with iron specific anemia, I recommend only naturized iron supplementation. Furthermore, iron supplementation is only necessary when a person presents with iron specific anemia. Iron supplementation should not be used to treat bone marrow specific anemia, as it provides no direct benefit for this condition.
I have found that many oncologists and medical doctors make iron supplementation recommendations solely based on a low hemoglobin/hematocrit in the complete blood count (CBC). Most cancer patients on chemotherapy develop bone-marrow anemia, not iron deficiency anemia, and should not be treated with iron at all.
How to differentiate between iron deficiency anemia and bone marrow/chronic disease anemia:
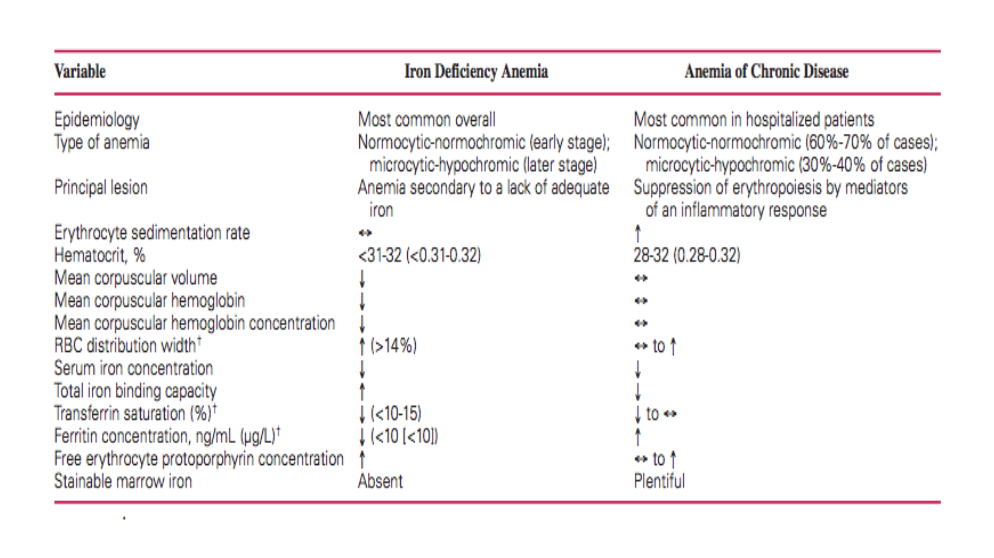
Natural Approach to Chemotherapy-Related Anemia
Chemotherapy-related bone marrow anemia should be treated with whole food bone marrow nourishing herbal formulations, such as “Immune Enhancing Bone Marrow Soup,” (a recipe given to all Mederi Center patients for bone marrow support and anemia), undenatured whey protein and colostrum and foods such as beet juice and black strap molasses. Undenatured whey protein and colostrum are rich in lactoferrin, a natural multifunctional protein known to boost the immune response by enhancing antioxidants and has been studied in human clinical trials in cancer patients during chemotherapy for its anti-microbial and anti-inflammatory effects.34-37 Additionally, undenatured whey protein supplementation has been shown to increase glutathione levels and improve nutritional status and immunity in cancer patients undergoing chemotherapy.38
In relation to the article presented here, the strongest association with worse outcome was in the patients supplementing with iron. There are several reasons that could explain this phenomenon. It is possible that the underlying existing condition that the supplement is used to treat, such as iron for anemia, could be related to breast cancer recurrence and death as opposed to the supplement itself. Another likely explanation is that the supplements were iron isolates and acted in a pro-oxidant fashion for one of the reasons listed above or the patients took dosages that were too high, which promoted the growth of cancer.
Example 2: Form and Quality Variation Among Vitamin E Supplements
Vitamin E is a term that can be used to refer to single isomers, racemic mixtures, or mixtures of any of several tocopherols (e.g., α-, β-, γ-, δ-tocopherols). Over-the-counter tocopherols can either be in the d-alpha (natural) form or the dl-alpha (synthetic) form. Research has demonstrated that the natural d-alpha form is far superior to the synthetic dl-alpha form.39-41 This study did not identify what form of tocopherol was used and simply described the compound as “vitamin E.”
At Mederi, we do not recommend commercial, isolated forms of tocopherols or tocopherols in general (other than Naturized tocopherol in a multi), and instead use tocotrienols, which have a substantial amount of research supporting their use in combination with chemotherapy. Tocotrienols are naturally occurring analogues of vitamin E and vary from tocopherols in several important ways. Tocotrienols have up to 60 times more powerful antioxidant properties in lipid biological systems than alpha-tocopherol.42 Cellular uptake of tocotrienols is up to 70 times higher, as their distinct chemical structure readily allows rapid absorption by body cells and organs.43
Accumulating evidence on the anti-cancer potency of vitamin E has shifted the focus from tocopherols to tocotrienols. Tocotrienols have the highest anti-cancer activitiy and target common molecular pathways involved in the inhibition of the cell cycle, the induction of apoptosis and autophagy, and the inhibition of invasion, metastasis, and angiogenesis.44
Additionally, these compounds were reported to specifically target the subpopulation of cancer stem cells, known to be deeply involved in the development of resistance to standard therapies.
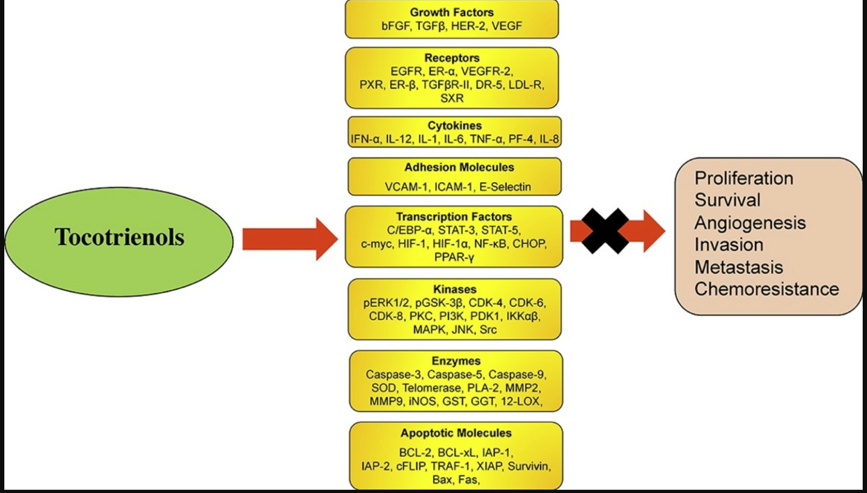
45
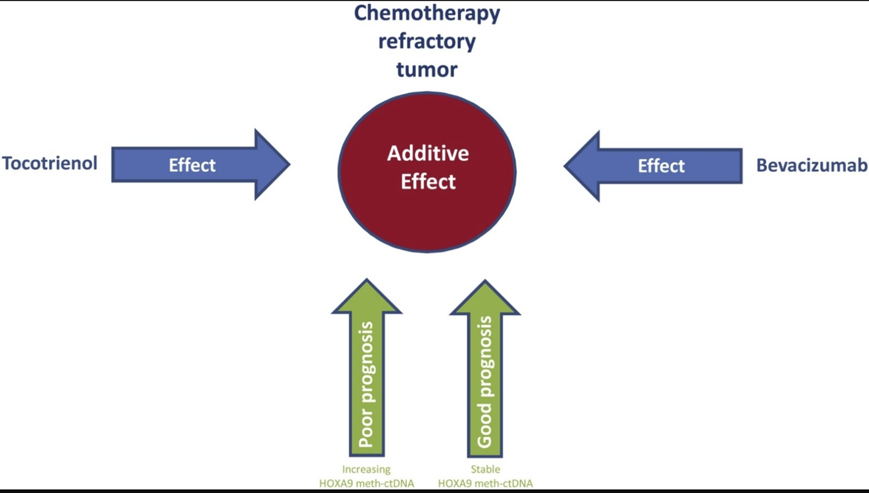
46
The combination of bevacizumab and tocotrienol is potent in chemotherapy refractory ovarian cancer.46 In addition, increasing lines of evidence have shown that tocotrienols can sensitize cancer cells to chemotherapeutic agents such as celecoxib, doxorubicin, erlotinib, gefitinib, gemcitabine, paclitaxel, statin, etc. (doxorubicin and paclitaxel were given in the study).47-49 Moreover, several clinical trials have confirmed the safety and tolerability of tocotrienols in humans.47,48
Carotenoids and Breast Cancer
A comprehensive prospective analysis (which included 18 prospective cohort studies) showed that women with higher circulating total carotenoid levels (α-carotene, β-carotene, lutein, zeaxanthin, lycopene) have a reduced incidence of breast cancer.49-53
At Mederi, we recommend supplements derived from whole-food sources of carotenoids, such as sea buckthorn oil (SBO). SBO contains an array of important nutrients and bioactive substances such as vitamins, carotenoids, flavonoids, polyunsaturated fatty acids, free amino acids and elemental components. Numerous clinical trials and scientific studies confirm the medicinal and nutritional value of SBO against cancer.52-58 The carotenoids found in SBO are unique, highly concentrated and are responsible for the rich orange color. The redox/antioxidant capacity of the SBO corresponds to the total and concentration of carotenoids.59,60
Vitamin D and Breast Cancer
In the four decades I have worked with cancer patients, I often find that many people tend to be deficient in the most critical nutrients for health, especially Vitamin D, as well as zinc and magnesium. In addition, certain chemotherapies deplete vital nutrients the body needs to maintain health (ie; platinum drugs and magnesium). This is why, in addition to the botanical formulations that are a core component of Mederi Care protocols, we often include these additional supplements, and in the Naturized form.
Research demonstrates that women with high, compared with low, plasma vitamin D (25 OH) levels have a reduced breast cancer risk61-63 and significant evidence links low levels of vitamin D with increased risk of recurrence and death in breast cancer patients.64
In multivariate regression models, patients who took vitamin D supplements reduced their risk of losing disease-free status by 64%. Median disease-free survival was 32.6 months among the 134 patients undergoing adjuvant chemotherapy who were taking any form of vitamin D supplementation versus a median of 25.5 months for the women not taking supplements (P = .022).65
Patients with breast cancer, as well as most other types of cancer, have low serum levels of zinc and high levels of copper.66 Overall, zinc appears to be the most important nutrient for immune system health and for supporting inflammation modulation.67-71
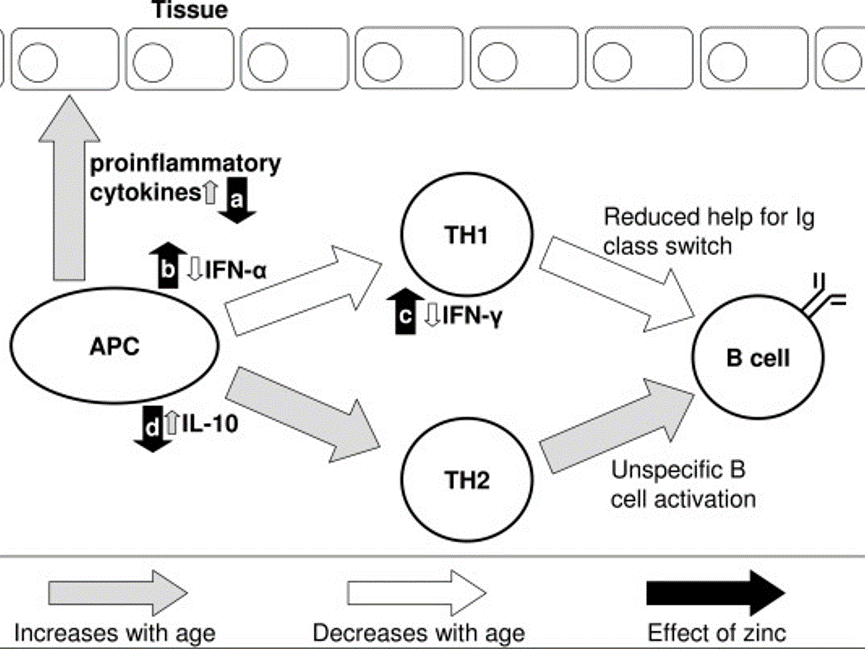
71
Chemotherapy Supportive Protocols Using the Mederi Care Approach
A large body of research demonstrates in vitro and in vivo synergy between natural compounds and anti-neoplastic drugs for cancer. However, it is imperative to seek expert advice in formulating an optimal chemotherapy supportive protocol for each person, based on their constitutional needs, tumor microenvironment, laboratory results, type and location of cancer, and the specific chemotherapy protocol.
One of the foundational principals of Mederi Care is that herbs and nutrients are generally best in balanced formulations that provide synergistic effects, similar to how a healthy diet includes a diversity of foods. Mederi protocols include formulations made up of multiple herbal extracts and botanical compounds based on traditional medicine and extensive clinical experience.
It has been my privilege to work with thousands of cancer patients for over four decades. At Mederi, our herbal protocols not only alleviate the symptoms experienced by cancer patients and improve their quality of life, but also prolong patient survival. I have many breast cancer patients I have worked with for over 10-20 years. While some herbal medicines are effective at reducing side-effects caused by antitumor drugs, others are able to exert therapeutic effects by upregulating immune responses, even in an immunosuppressive tumor microenvironment.72
The Unique Benefits of Natural Compounds in Cancer Therapies
Herbal extracts and natural compounds target molecular pathways both distinct from and in common with cancer drugs, thus having the potential to enhance their therapeutic effects. Herbs contain a large variety of active phytochemicals such as carotenoids, flavonoids, ligands, polyphenolics, terpenoids, sulfides, lignans and plant sterols. These phytochemicals show more structural diversity, bioactivity and complexity than compounds in synthetic drug libraries, and have the ability to inhibit some targets considered “undruggable,” such as protein-protein interactions. The mechanisms of action of phytochemicals are inherently biologically relevant since they are mostly secondary metabolites or signaling molecules.
The synergistic effect herbal medicines can provide to cancer drugs is illustrated below by the combination of the natural compound Artemisinin (from Artemisia annua) and the biological drug Imatinib (Gleevac).73
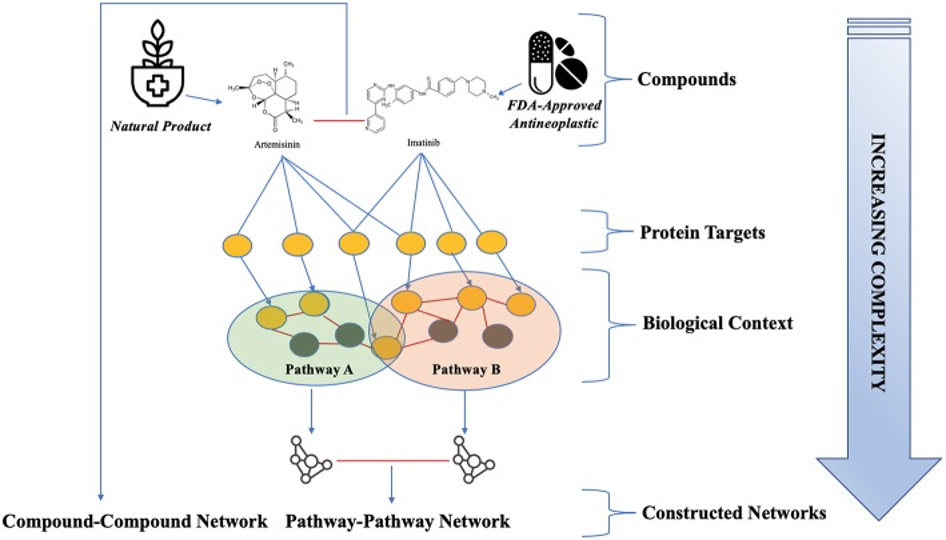
73
Abundant evidence has been collected for beneficial effects of flavonoids, carotenoids, phenolic acids, and organosulfur compounds affecting a number of cancer-related pathways. These natural compounds may positively affect processes of cell signaling, cell cycle regulation, oxidative stress response, and inflammation.74
Cancer stem cells (CSCs) are characterized as a subpopulation of cancer cells with aberrant regulation of self-renewal, proliferation or apoptosis leading to cancer progression, invasiveness, metastasis formation, and therapy resistance. Anticancer effects of natural compounds are also uniquely directed to target CSCs, of which chemotherapy does not.75
There is strong evidence that many natural compounds reduce chemotherapy and radiotherapy-induced oral mucositis, gastrointestinal toxicity, hepatotoxicity, nephrotoxicity, hematopoietic system injury, cardiotoxicity, and neurotoxicity.76
Natural compounds, such as curcumin, lycopene, capsaicin, and ursolic acid possess anti-inflammatory, anti-metastatic, anti-proliferative, anti-angiogenic, and anti-cancer properties in breast cancer.77
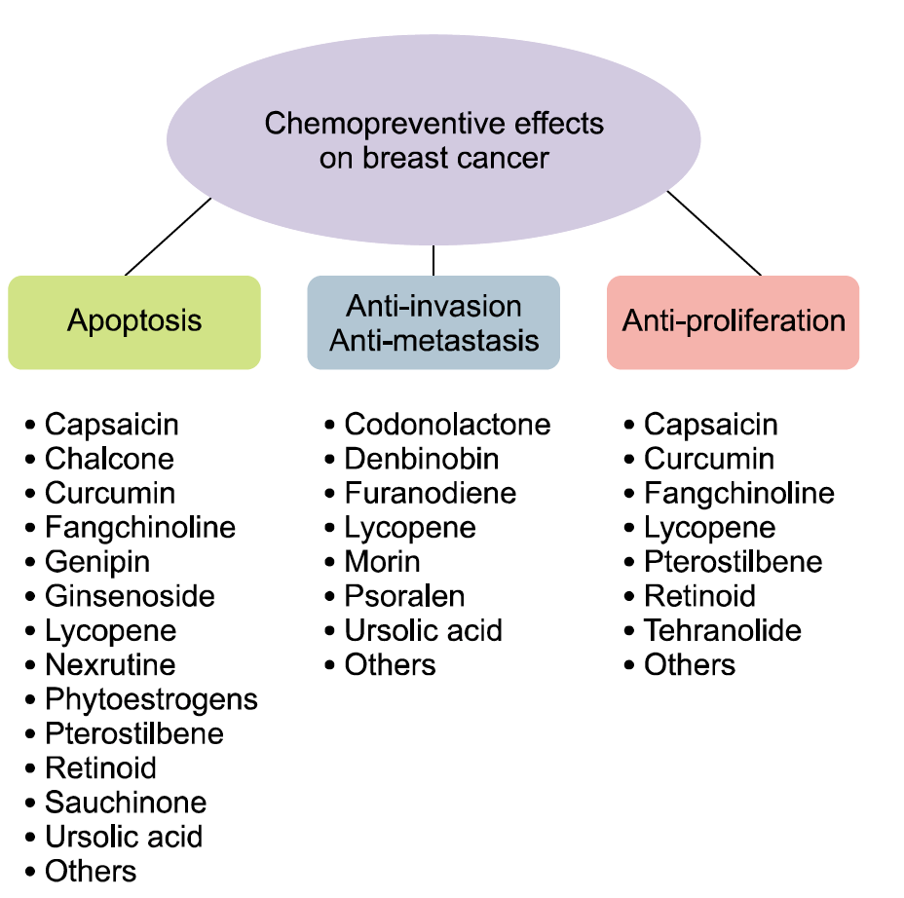
77
What Does the Data Really Say About Antioxidant Use and Chemotherapy?
As far as antioxidant research goes, robust meta-analysis studies have concluded that antioxidant use with chemotherapy offers an improvement in treatment response and survival. According to a large systematic review of the use of antioxidants during chemotherapy, “none of the trials reported evidence of significant decreases in efficacy from antioxidant supplementation during chemotherapy. Many of the studies indicated that antioxidant supplementation resulted in either increased survival times, increased tumor responses, or both, as well as fewer toxicities than controls; however, lack of adequate statistical power was a consistent limitation.”78-83
The Journal of the National Cancer Institute published a paper (Nov 5, 2008) entitled, “Should Supplemental Antioxidant Administration Be Avoided During Chemotherapy and Radiation Therapy?”
“Cancer patients have been told not to take supplemental antioxidants during treatment because a single interview in The New York Times in 1997 that was not based on published scientific work and a single research paper involving mouse cells, along with a press release by its author in 1999, which led to the erroneous notion that vitamin C interferes with chemotherapy and radiation in humans. This notion soon applied to all antioxidants as physicians, patients, the media, the American Cancer Society, and scores of websites took the same position without reviewing the scientific evidence.”
The findings are clear and consistent over decades: “Since the 1970s, 280 peer-reviewed in vitro and in vivo studies, including 50 human studies involving 8,521 patients, 5,081 of whom were given nutrients, have consistently shown that non-prescription antioxidants and other nutrients do not interfere with therapeutic modalities for cancer.”
“Furthermore, they enhance the killing of therapeutic modalities for cancer, decrease their side effects, and protect normal tissue. In 15 human studies, 3,738 patients who took non-prescription antioxidants and other nutrients actually had increased survival.”79,80
In a 10-year retrospective survival study, the authors investigated herbal medicine and vitamin therapy in a consecutive case series of all non-small-cell lung cancer patients. Herbal medicine with vitamins, combined with conventional therapy, improved survival in stages IIIA, IIIB, and IV, compared with conventional therapy alone.81
A study published in December 2010, in Cancer Epidemiology, Biomarkers & Prevention found no evidence that the use of vitamins during the first 6 months after a diagnosis of breast cancer adversely affected outcomes. In fact, it found quite the opposite: vitamin use appeared to have a beneficial effect among patients with breast cancer who underwent chemotherapy. Vitamin use —and the use of vitamins C and E in particular—appeared to be associated with reduced risk for mortality and recurrence.
“Patients who used antioxidants (vitamin E, vitamin C, multivitamins) had an 18% reduction in their mortality risk, and the risk for recurrence was decreased by 22%. This association was observed whether vitamin use was concurrent or non-concurrent with chemotherapy.”
Although complete information was not available for dosages taken,
approximately 85% of vitamin C users took 400 mg/day or less and 99% of vitamin
E users took 400 mg/day or less.82
Another study evaluated the association of vitamin supplement use in the first six-months after breast cancer diagnosis and during cancer treatment with total mortality and recurrence. This was a cohort study that included women who were diagnosed with early stage, primary breast cancer from 1997 to 2000, who enrolled on average 2 years post diagnosis. Baseline data was collected on antioxidant supplement use since diagnosis and other factors.
“Antioxidant supplement use after diagnosis was reported by 81% of women. Among antioxidant users, frequent use of vitamin C and vitamin E was associated with a decreased risk of BC recurrence; and vitamin E use was associated with a decreased risk of all-cause mortality. Conversely, frequent use of combination carotenoids was associated with a non-significant increased risk of death from BC and all-cause mortality.”83
In addition, it is well known that poor antioxidant status and high oxidative stress are associated with breast cancer risk.84
Supplement Dosages are a Critical Factor
Everything has the potential to be harmful if the dose is too high, even water, which in excess can cause drowning. As far as antioxidants, an animal study carried out by Swedish researchers showed that the harm from antioxidants was dose dependent.85
“All things are poison and not poison; only the dose makes a thing poison,” wrote Paracelsus. It is the dose that makes the medicine, and the dose that makes the poison.

The dose–response relationship, or exposure–response relationship, describes the change in effect caused by differing levels of exposure to a stressor (or food or supplement) after a certain exposure time. Everything has a biphasic ‘U-Curve’ effect, which means while too little or too much of something can result in a negative outcome, the correct amount will have a positive effect.
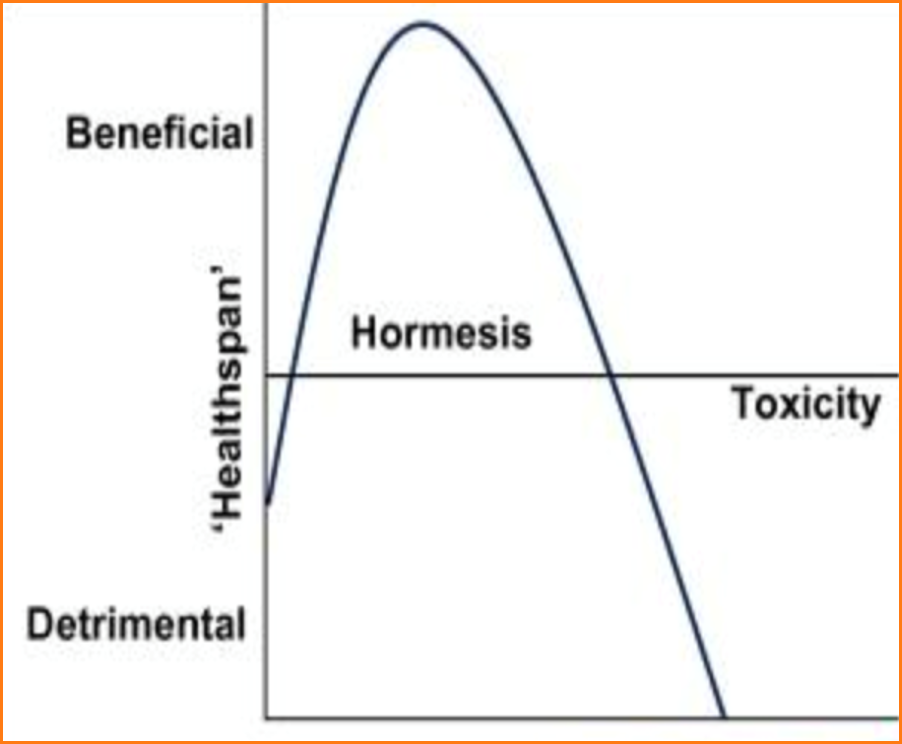
86
For example, consider exercise. People with low levels of physical activity are more prone to oxidative stress, and a lack of exercise makes you more susceptible to a variety of diseases. But the same is true of people who over-exercise. Regular exercise, at both low and high intensities with periods of rest in-between, lowers oxidative stress and improves longevity and brain health. Exercise itself causes oxidative stress, especially high intensity exercise like lifting weights or intensive cardio. However, it also triggers adaptations that increase mitochondrial density and biogenesis through mitochondrial hormesis.87,88
The Potential for Future Research on Chemotherapy Supportive Herbal Protocols
In summary, in light of the potential confounding factors and biases identified in this study, as I said earlier it is “much ado about nothing.” I find it curious that a study with such low-level evidence has garnered so much attention, while the many robust, high-quality studies in favor of antioxidants have gone unnoticed. Unfortunately, this is likely because there is tremendous bias in both the medical establishment and the mainstream news, both of which paint a negative outlook on the benefits of supplements on health.
The Mederi Foundation has been working with cancer institutions to conduct meaningful clinical trials that could significantly improve people’s lives. One of those clinical trials is with the Ohio State University’s (OSU) James Cancer Center in patients with advanced breast cancer. Although we have made enormous progress toward this worthwhile endeavor, we have not been able to get the final IRB approval because of the complexity of ingredients and concerns over herb/nutrient interactions, despite the over-whelming evidence we have presented to support the likelihood that potential interactions will be positive. This has also been the case with other attempts made to conduct meaningful clinical trials with other institutions, such as with the Children’s Hospital of Orange County to support children on chemotherapy drugs who get mouth sores (stomatitis / mucositis).
With regards to the OSU study, we specifically picked a population of advanced (Stage 4) breast cancer patients who had already failed multiple chemotherapies and other treatments. The details we worked through along the way were comprised of everything from committee, board, pharmacy, and FDA approvals, to the logistics of carrying out and managing a study of this magnitude. Of paramount importance was ensuring the safety of the participants, which involved extensive preparation of safety data, eligibility criteria, informed consent, and disclosures. All of this was coordinated among several departments by a team of expert research physicians who also see patients on a full-time basis.
In May of 2015, we surpassed the first major milestone and our study protocol was approved by the Clinical Scientific Review Committee (CSRC). This approval however, came with stipulations that involved determining how the extensive supplement regimen would be dispensed by the pharmacy, yet another detailed undertaking. Once these logistics were determined, the protocol was submitted to the Institutional Review Board (IRB). Following their review, concerns arose over potential herb-drug interactions. Potential herb-drug interactions are a common point of contention between conventional doctors and holistic practitioners who recommend ‘biologics’ (herbal and nutritional supplements). Despite our extensive expertise in this area, and the safety parameters incorporated into the protocol for monitoring changes in drug efficacy and elimination, it was decided that a different drug regimen would need to be explored, one that did not involve drugs metabolized via liver enzyme pathways.
At present, it has been agreed that the protocol will be developed for women with Her2/Neu positive breast cancer being treated with Herceptin. This involves numerous changes to the current protocol. The principal investigators and the chair of the IRB are confident that this approach will lead to the final approval.
We will never stop trying to launch this and other trials in the hopes that Mederi Medicine, a unitive approach to cancer, will be given a chance to prove that it significantly improves cancer patients’ lives and survival. Please keep this breakthrough initiative for women with breast cancer in your prayers.
For More Blog Posts On This Topic:
The Pursuit of Truth in Medicine
Botanical Compounds in Cancer Combination Therapies
Are “Wonder Drugs” the Answer to Curing Cancer?
Current Research into Botanicals and Cancer
Botanical Medicine: How Herbs Represent A Symphony in Harmony Against Cancer
Integrating Botanical Medicine in Cancer Therapies
References:
- Christine B. Ambrosone, PhD1; Gary R. Zirpoli, PhD2; Alan D. Hutson, PhD1; William E. McCann1; Susan E. McCann, PhD, RD1; William E. Barlow, PhD3; Kara M. Kelly, MD1; Rikki Cannioto, PhD, EdD1; Lara E. Sucheston-Campbell, PhD4; Dawn L. Hershman, MD5; Joseph M. Unger, PhD3; Halle C.F. Moore, MD6; James A. Stewart, MD7; Claudine Isaacs, MD8; Timothy J. Hobday, MD9; Muhammad Salim, MD10; Gabriel N. Hortobagyi, MD11; Julie R. Gralow, MD12; George T. Budd, MD6; and Kathy S. Albain, MD13 Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients With Breast Cancer Enrolled in a Cooperative Group Clinical J Clin Oncol. 2019 Dec 19:JCO1901203. doi: 10.1200/JCO.19.01203.
- Dugald Seely, ND, MSc, FABNO, Athanasios Psihogios, ND, and Heather Wright, ND, FABNO, Dietary Supplement Use During Chemotherapy, Review of a recent high-profile publication in the Journal of Clinical Oncology, Natural Medicine Journal, March 2020 Vol. 12 Issue 3.
- Akiko Nakayama,1,* Karen P. Alladin,2,* Obianuju Igbokwe,2 and Jeffrey D. White; Systematic Review: Generating Evidence-Based Guidelines on the Concurrent Use of Dietary Antioxidants and Chemotherapy or Radiotherapy, Cancer Invest. 2011 Dec; 29(10): 655–667. doi: 10.3109/07357907.2011.626479.
- https://thelogicofscience.com/2016/01/12/the-hierarchy-of-evidence-is-the-studys-design-robust/, Posted on January 12, 2016, retrieved 3/4/2020.
- Zaman S , Lippman SI, Zhao X, Broach JR. How Saccharomyces responds to nutrients, Annu Rev Genet. 2008;42:27-81. doi: 10.1146/annurev.genet.41.110306.130206.
- Bach, A., C. Iglesias, and M. Devant. 2007. Daily rumen pH pattern of loose housed dairy cattle as affected by feeding pattern and live yeast supplementation. Animal Feed Science and Technology 136(1-2):146- 153.
- Chaucheyras-Durand, F.,and G. Fonty, G. 2001.Establishment of cellulolytic bacteria and development of fermentation activities in the rumen of gnotobiotically-reared lambs receiving the microbial additive Saccharomyces cerevisiae CNCM I-1077.Repord.Nutr. Dev. 41, 57- 68.
- Chaucheyras-Durand, F., and G. Fonty, G. 2002. Influence of a probiotic yeast (Saccharomyces cerevisiae CNC I-1077) on microbial colonization and fermentation in the rumen of newborn lambs. Microb. Ecol. Health Dis. 14:30-36.
- Chaucheyras-Durand, F., N. D. Walker, and A. Bach. 2008. Effects of active dry yeasts on the rumen microbial ecosystem: Past, present and future. Animal Feed Science and Technology 145(1-4):5-26.
- Palma ML, Zamith-Miranda D, Martins FS, Bozza FA, Nimrichter L, Montero-Lomeli M, Marques ET Jr, Douradinha B. Probiotic Saccharomyces cerevisiae strains as biotherapeutic tools: is there room for improvement? Appl Microbiol Biotechnol. 2015 Aug;99(16):6563-70. doi: 10.1007/s00253-015-6776-x.
- Michael Hoppe ,Gunilla Önning, Lena Hulthén, Freeze-dried Lactobacillus plantarum 299v increases iron absorption in young females—Double isotope sequential single-blind studies in menstruating women, PLoS One. 2017 Dec 13;12(12):e0189141. doi: 10.1371/journal.pone.0189141. eCollection 2017..
- Hoppe M, Önning G, Berggren A, Hulthén L Probiotic strain Lactobacillus plantarum 299v increases iron absorption from an iron-supplemented fruit drink: a double-isotope cross-over single-blind study in women of reproductive age..Br J Nutr. 2015 Oct 28; 114(8):1195-202.
- Bering S, Suchdev S, Sjøltov L, Berggren A, Tetens I, Bukhave K., A lactic acid-fermented oat gruel increases non-haem iron absorption from a phytate-rich meal in healthy women of childbearing age. Br J Nutr. 2006 Jul; 96(1):80-5.
- Elizabeth K Lund, S Gabrielle Wharf, Susan J Fairweather-Tait, Ian T Johnson, Oral ferrous sulfate supplements increase the free radical–generating capacity of feces from healthy volunteers1,2,3, Am J Clin Nutr February 1999, vol. 69 no. 2 250-255
- Elizabeth K Lund, S Gabrielle Wharf, Susan J Fairweather-Tait, Ian T Johnson, Oral ferrous sulfate supplements increase the free radical–generating capacity of feces from healthy volunteers1,2,3, Am J Clin Nutr February 1999, vol. 69 no. 2 250-255
- Joseph E. Baggott and Tsunenobu Tamura, Homocysteine, Iron and Cardiovascular Disease: A Hypothesis, Nutrients. 2015 Feb; 7(2): 1108–1118, Published online 2015 Feb 6. doi: 10.3390/nu7021108.
- Liangfu Zhou, Bin Zhao, Lixiu Zhang, Shenghang Wang, Dandan Dong, Huanhuan Lv, Peng Shang Alterations in Cellular Iron Metabolism Provide More Therapeutic Opportunities for Cancer Int J Mol Sci. 2018 May; 19(5): 1545. Published online 2018 May 22. doi: 10.3390/ijms19051545
- Liang fu Zhou 1 , Bin Zhao 1 , Lixiu Zhang 1 , Shenghang Wang 1 , Dandan Dong 1 , Huanhuan Lv, Peng Shang 2,3, Alterations in Cellular Iron Metabolism Provide More Therapeutic Opportunities for Cancer, Int. J. Mol. Sci. 2018, 19, 1545; doi:10.3390/ijms19051545.
- Christa Pfeifhofer-Obermair, Piotr Tymoszuk, et. al., Iron in the Tumor Microenvironment—Connecting the Dots, Front. Oncol., 26 November 2018 | https://doi.org/10.3389/fonc.2018.00549
- Vicky C Chang 1 2, Michelle Cotterchio 1 2, Susan J Bondy 1, Joanne Kotsopoulos 1 3 Iron Intake, Oxidative Stress-Related Genes, and Breast Cancer Risk, Int J Cancer, 2020 Feb 5, Online DOI: 10.1002/ijc.32906.
- Nimeus E, Malmstrom J, Johnsson A, Marko-Varga G, Ferno M. Proteomic analysis identifies candidate proteins associated with distant recurrences in breast cancer after adjuvant chemotherapy. J Pharm Biomed Anal. (2007) 43:1086–93. doi: 10.1016/j.jpba.2006.09.019.
- Jiang XP, Elliott RL, Head JF. Manipulation of iron transporter genes results in the suppression of human and mouse mammary adenocarcinomas. Anticancer Res. (2010) 30:759–65.
- Zhang S, Chen Y, Guo W, Yuan L, Zhang D, Xu Y, et al. Disordered hepcidin-ferroportin signaling promotes breast cancer growth. Cell Signal. (2014) 26:2539–50. doi: 10.1016/j.cellsig.2014.07.029.
- Boult J, Roberts K, Brookes MJ, Hughes S, Bury JP, Cross SS, et al. Overexpression of cellular iron import proteins is associated with malignant progression of esophageal adenocarcinoma. Clin Cancer Res. (2008) 14:379–87. doi: 10.1158/1078-0432.CCR-07-1054.
- Brookes MJ, Hughes S, Turner FE, Reynolds G, Sharma N, Ismail T, et al. Modulation of iron transport proteins in human colorectal carcinogenesis. Gut (2006) 55:1449–60. doi: 10.1136/gut.2006.094060.
- Ludwig H, Evstatiev R, Kornek G, Aapro M, Bauernhofer T, Buxhofer-Ausch V, et al. Iron metabolism and iron supplementation in cancer patients. Wien Klin Wochenschr. (2015) 127:907–19. doi: 10.1007/s00508-015-0842-3.
- 27) Steurer M, Wagner H, Gastl G. Prevalence and management of anaemia in haematologic cancer patients receiving cyclic nonplatinum chemotherapy: results of a prospective national chart survey. Wien Klin Wochenschr. (2004) 116:367–72. doi: 10.1007/BF03040915.
- Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer (2001) 91:2214–21. doi: 10.1002/1097-0142(20010615)91:12 < 2214::AID-CNCR1251>3.0.CO;2-P.
- Knight K, Wade S, Balducci L., Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. 2004 Apr 5; 116 Suppl 7A():11S-26S.
- Aratirika Chakraborthy, Pratibha Ramani 1, Herald Justin Sherlin, Priya Premkumar, Anuja Natesan; Antioxidant and Pro-Oxidant Activity of Vitamin C in Oral Environment; Indian J Dent Res, 25 (4), 499-504 Jul-Aug 2014.
- Gang Chen, T M S Chang 2 Dual Effects Include Antioxidant and Pro-Oxidation of Ascorbic Acid on the Redox Properties of Bovine Hemoglobin, Artif Cells Nanomed Biotechnol, 46 (sup2), 983-992, 2018.
- Podmore ID, Griffiths, HR, Herbert KE, et al. Vitamin C exhibits pro-oxidant properties. Nature 1998;392:559.
- Loots, Deirdré; Oosthuizen, Welma; Pieters, Marlien; Spies, Christelle; Vorster, Hester H, Foodstate vitamin C complex may beneficially affect haemostasis and fibrin network structure in hyperlipidaemic patients, Blood Coagulation & Fibrinolysis: December 2004 – Volume 15 – Issue 8 – pp 677-685.
- Namrata Anand, Rupinder K Kanwar, Mohan Lal Dubey, R K Vahishta, Rakesh Sehgal, Anita K Verma, Jagat R Kanwar, Effect of lactoferrin protein on red blood cells and macrophages: mechanism of parasite–host interaction, Drug Des Devel Ther. 2015; 9: 3821–3835. Published online 2015 Jul 27. doi: 10.2147/DDDT.S77860.
- Bumrungpert A, et al. Whey Protein Supplementation Improves Nutritional Status, Glutathione Levels, and Immune Function in Cancer Patients: A Randomized, Double-Blind Controlled Trial. J Med Food 2018 – Clinical Trial. PMID 29565716.
- Moastafa TM, et al. Study on the Therapeutic Benefit on Lactoferrin in Patients with Colorectal Cancer Receiving Chemotherapy. Int Sch Res Notices 2014.
- Wang A, et al. Effect of lactoferrin on taste and smell abnormalities induced by chemotherapy: a proteome analysis. Food Funct 2018 – Clinical Trial. PMID 301821.
- Filipe J Teixeira , Heitor O Santos, Scott L Howell, Gustavo D Pimentel, Whey Protein in Cancer Therapy: A Narrative Review, Pharmacol Res, 144, 245-256 Jun 2019, DOI: 10.1016/j.phrs.2019.04.019.
- Kiyose C, et al. Biodiscrimination of
alpha-tocopherol stereoisomers in humans
after oral administration, Am J Clin Nutr 1997 (Mar); 65 (3): 785-9. - Burton GW, et al.Human plasma and tissue
alpha-tocopherol concentrations in response to
supplementation with deuterated natural and synthetic vitamin E, Am J Clin Nutr 1998; 67: 669-84. - Traber MG, et al. Synthetic as compared
with natural vitamin E is preferentially
excreted as a-CEHC in human urine: studies using deuterated a-tocopheryl acetate
FEBS Letters 1998 (Oct 16); 437:145-8. - Serbinova E, Kagan V, Han D & Packer L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha tocotrienol. Free Radic Biol Med 1991;10(5).
- Saito Y, Yoshida Y, Nishio K, et al. Characterization of cellular uptake and distribution of vitamin E. Ann N Y Acad Sci 2004;1031.
- Constantinou C, et al. Vitamin E and cancer: an update on the emerging role of γ and δ tocotrienols. Eur J Nutr 2019 – Review. PMID 31016386.
- Montagnani Marelli M, Marzagalli M, Fontana F, Raimondi M, Moretti RM, Limonta P. Anticancer properties of tocotrienols: A review of cellular mechanisms and molecular targets. J Cell Physiol. 2018 Aug 1. doi: 10.1002/jcp.27075. Review.
- Caroline Brenner ThomsenacRikke Fredslund AndersenbKarina, Dahl SteffensenacParvin AdimiaAnders Jakobsen, Delta tocotrienol in recurrent ovarian cancer. A phase II trial, ScienceDirect, Pharmacological Research, Volume 141, March 2019, Pages 392-396.
- Bethsebie Lalduhsaki Sailo, Kishore Banik, Ganesan Padmavathi, Monisha Javadi, Devivasha Bordoloi, Ajaikumar B. Kunnumakkara, Tocotrienols: The promising analogues of vitamin E for cancer Therapeutics Pharmacological Research, Volume 130, April 2018, Pages 259-272
- Takahiro Eitsuka 1, Naoto Tatewaki, Hiroshi Nishida, Kiyotaka Nakagawa and Teruo Miyazawa, Synergistic Anticancer Effect of Tocotrienol Combined with Chemotherapeutic Agents or Dietary Components: A Review, Int. J. Mol. Sci. 2016, 17, 1605; doi:10.3390/ijms17101605.
- X Zhang et al. Carotenoid Intakes and Risk of Breast Cancer Defined by Estrogen Receptor and Progesterone Receptor Status: A Pooled Analysis of 18 Prospective Cohort Studies;. Am J Clin Nutr 95 (3), 713-25. Mar 2012. PMID 22277553.
- A Heather Eliassen, et. al., Circulating Carotenoids and Risk of Breast Cancer: Pooled Analysis of Eight Prospective Studies, J Natl Cancer Inst. 2012 Dec 19;104(24):1905-16. doi: 10.1093/jnci/djs461.
- Prakash P, Russell RM, and Krinsky NI, In vitro inhibition of proliferation of estrogen-dependent and estrogen- independent human breast cancer cells treated with carotenoids or retinoids, Journal of Nutrition, 131:1574-80, 2001.
- Simon MS, Djuric Z, Dunn B, Stephens D, Lababidi S, and Heilbrun LK, An Evaluation of Plasma Antioxidant Levels and the Risk of Breast Cancer: A Pilot Case Control Study, Breast Journal, 6: 388-395, 2000.
- Toniolo P, Van Kappel AL, Akhmedkhanov A, Ferrari P, Kato I, Shore RE, and Riboli E, Serum carotenoids and breast cancer. American Journal of Epidemiology, 153:1142-7, 2001.
- Olas B, Skalski B, Ulanowska K. The Anticancer Activity of Sea Buckthorn [Elaeagnus rhamnoides (L.) A. Nelson]. Front Pharmacol. 2018 Mar 15;9:232. doi: 10.3389/fphar.2018.00232. eCollection 2018. Review.
- Olas B. The beneficial health aspects of sea buckthorn (Elaeagnus rhamnoides (L.) A.Nelson) oil. J Ethnopharmacol. 2018 Mar 1;213:183-190. doi: 10.1016/j.jep.2017.11.022. Epub 2017 Nov 21.
- Yasukawa K, Kitanaka S, Kawata K, Goto K. Anti-tumor promoters phenolics and triterpenoid from Hippophae rhamnoides. Fitoterapia. 2009 Apr;80(3):164-7. Epub 2009 Jan 23.
- Sun B, Zhang P, Qu W, Zhang X, Zhuang X, Yang H. Study on effect of flavonoids from oil-removed seeds of Hippophae rhamnoides on inducing apoptosis of human hepatoma cell]. Zhong Yao Cai. 2003 Dec;26(12):875-7.
- Gao X; Ohlander M; Jeppsson N; Bjork L; Trajkovski V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J Agric Food Chem 2000 May;48(5):1485-90.
- Zielińska A, Nowak I, Abundance of active ingredients in sea-buckthorn oil. Lipids Health Dis. 2017 May 19;16(1):95. doi: 10.1186/s12944-017-0469-7.
- Kasparaviciene G, Briedis V, Ivanauskas L. [Influence of sea buckthorn oil production technology on its antioxidant activity] Medicina (Kaunas). 2004;40(8):753-7.
- Eliassen AH1, Warner ET2, Rosner B3, Collins LC4, Beck AH4, Quintana LM4, Tamimi RM5, Hankinson SE6. Plasma 25, Hydroxyvitamin D and Risk of Breast Cancer in Women Followed over 20 Years, Cancer Res. 2016 Sep 15;76(18):5423-30. doi: 10.1158/0008-5472.CAN-16-0353.
- Machado MRM, de Sousa Almeida-Filho B, De Luca Vespoli H, Schmitt EB, Nahas-Neto J, Nahas EAP. Low pretreatment serum concentration of vitamin D at breast cancer diagnosis in postmenopausal women. Menopause. 2018 Sep 17. doi: 10.1097/GME.0000000000001203.
- THERESA SHAO, PAULA KLEIN, MICHAEL L. GROSSBARD, Vitamin D and Breast Cancer, The Oncologist,2012;17:36 – 45
- Rose A, Elser C, Ennis M, Goodwin P; Blood levels of vitamin D and early stage breast cancer prognosis: a systematic review and meta-analysis; Breast Cancer Research and Treatment (Oct 2013).
- Morrison, Alex, Vitamin D Supplementation Improves Outcomes in Women With HER2-Positive Breast Cancer, SAN ANTONIO, Tex — December 17, 2013, [Presentation title: Improved Clinical Outcomes Associated With Vitamin D Supplementation During Adjuvant Chemotherapy in Patients With HER2+ Non-Metastatic Breast Cancer. Abstract P609-02]
- Ajayi GO. Copper and zinc concentrations in Nigerian women with breast cancer, Eur J Gynaecol Oncol. 2011;32(3):307-8.
- Haase H, Rink L. Multiple impacts of zinc on immune function. Metallomics. 2014 Feb 17.
- Kloubert V, Blaabjerg K, Dalgaard TS, Poulsen HD, Rink L, Wessels I. Influence of zinc supplementation on immune parameters in weaned pigs. J Trace Elem Med Biol. 2018 Sep;49:231-240. doi: 10.1016/j.jtemb.2018.01.006.
- Ananda S. Prasad, Zinc: Mechanisms of Host Defense, The Journal of Nutrition, Volume 137, Issue 5, May 2007, Pages 1345-1349.
- The Authors. Production and Hosting by Elsevier B.V. on behalf of King Saud University, Natural cures for breast cancer treatment, Saudi Pharmaceutical Journal (2016) 24, 233–240.
- Smailhodzic D, van Asten F, et al. The immune system and the impact of zinc during aging. Immunity & Aging. 2009. 6:9. PLOS ONE. 2014 Nov. 9(11):1-10. 24.
- Annie A Wu, Virginia Drake, Huai-Shiuan Huang,3 ShihChi Chiu,3 and Lei Zheng, Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells, Oncoimmunology. 2015 Jul; 4(7): e1016700., Published online 2015 Apr 1. doi: 10.1080/2162402X.2015.1016700.
- Chamberlin SR, Blucher A, Wu G, Shinto L, Choonoo G, Kulesz-Martin M, McWeeney S. Natural Product Target Network Reveals Potential for Cancer Combination Therapies. Front Pharmacol. 2019 May 31;10:557. doi: 10.3389/fphar.2019.00557. eCollection 2019.
- A. Kapinova, P. Kubatka, O. Golubnitschaja, M. Kello, P. Zubor, P. Solar, M. Pec, Dietary phytochemicals in breast cancer research: anticancer effects and potential utility for effective chemoprevention, Environ Health Prev Med. 2018; 23: 36. Published online 2018 Aug 9. doi: 10.1186/s12199-018-0724-1.
- Liskova A, Kubatka P, Samec M, Zubor P, Mlyncek M, Bielik T, Samuel SM, Zulli A, Kwon TK, Büsselberg D. Dietary Phytochemicals Targeting Cancer Stem Cells, Molecules. 2019 Mar 4; 24(5). Epub 2019 Mar 4.
- Qing-Yu Zhang, Fei-Xuan Wang, Ke-Ke Jia and Ling-Dong Kong, Natural Product Interventions for Chemotherapy and Radiotherapy-Induced Side Effects, Frontiers in Pharmacology | www.frontiersin.org 1 November 2018 | Volume 9 | Article 1253.
- Eun-Yi Ko and Aree Moon: Natural Products for Chemoprevention of Breast Cancer, J Cancer Prev 2015;20:223-231.
- Block KI, Koch AC, Mead MN, Tothy PK, Newman RA, Gyllenhaal C. Impact of Antioxidant Supplementation on Chemotherapeutic Efficacy: A Systematic Review of the Evidence From Randomized Controlled Trials; Int J Cancer. 2008 Sep 15; 123(6):1227-39.
- Charles B. Simone II, MD; Nicole L. Simone, MD; Victoria Simone, RN; Charles B. Simone, MD. ANTIOXIDANTS AND OTHER NUTRIENTS DO NOT INTERFERE WITH CHEMOTHERAPY OR RADIATION THERAPY AND CAN INCREASE KILL AND INCREASE SURVIVAL, PART 1 and 2. Altern Ther Health Med. Jan-Feb, and Mar-Apr, 2007;13(1):22-28; 13(2): 40-7.)
- Simone CB, Simone NL, Simone CB II. Oncology Care Augmented with Nutritional and Lifestyle Modification. J Ortho Mol Med. 1997; 12(4): 197-206. Simone CB. Cancer and Nutrition, A Ten Point Plan for Prevention and Cancer Life Extension. Princeton Institute. 2006.
- Michael McCulloch, LAc, MPH, PhD, Michael Broffman, LAc, Mark van der Laan, PhD, … Lung Cancer Survival With Herbal Medicine and Vitamins in a Whole-Systems Approach: Ten-Year Follow-up Data Analyzed With Marginal Structural Models and Propensity Score, August 8th, 2011, https://doi.org/10.1177/1534735411406439.
- Volkan I. Sayin, Mohamed X. Ibrahim1, Erik Larsson3, Jonas A. Nilsson1,4, Per Lindahl and Martin O. Bergo, Antioxidants Accelerate Lung Cancer Progression in Mice, Science Translational Medicine 29 Jan 2014: Vol. 6, Issue 221, pp. 221ra15 DOI: 10.1126/scitranslmed.3007653.
- Sarah Nechuta,1 Wei Lu,2 Zhi Chen,1 Ying Zheng,2 Kai Gu,2 Hui Cai,1 Wei Zheng,1 and Xiao Ou Shu1, Vitamin supplement use during breast cancer treatment and survival: a prospective cohort study, Cancer Epidemiol Biomarkers Prev. 2011 Feb; 20(2): 262–271. Published online 2010 Dec 21. doi: 10.1158/1055-9965.EPI-10-1072.
- Greenlee H, Kwan ML, Kushi LH, Song J, Castillo A, Weltzien E, Quesenberry CP Jr, Caan, Vitamin supplement use during breast cancer treatment and survival: a prospective cohort study, BJ. Cancer. 2012 Apr 15; 118(8):2048-58. Epub 2011 Sep 27.
- Sharhar S, Normah H, Fatimah A, Fadilah RN, Rohi GA, Amin I, Cham BG, Rizal RM, Fairulnizal MN. Antioxidant intake and status, and oxidative stress in relation to breast cancer risk: a case-control study, Asian Pac J Cancer Prev. 2008 Apr-Jun;9(2):343-50.
- Mao, Jacqueline Franke , Hormesis in Aging and Neurodegeneration—A Prodigy Awaiting Dissection, Int. J. Mol. Sci. 2013, 14, 13109-13128; Rattan S. Hormesis in aging. Ageing Res Rev. 2008; 7:63-78.
- Radak, Zsolt; Chung, Hae Y.; Koltai, Erika; Taylor, Albert W.; Goto, Sataro (2008). “Exercise, oxidative stress and hormesis”. Ageing Research Reviews. 7 (1): 34.
- Ristow, Michael; Zarse, Kim (2010). “How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis)”. Experimental Gerontology. 45 (6): 410.