Over the past 30 years, the world has seen an increase in the rates of cancer in patients younger than 50. Many physicians and scientists around the world have joined in the great detective story to untangle the causes of this complex medical mystery.
Since the pandemic began, oncologists have been noticing a disturbing rise in cancer cases, including rare cancers among younger adults. Early data from national sources and some large cancer institutions also suggests that there has been an increase in aggressive late-stage cancers.
“We started noticing some very unusual patterns,” said Kashyap Patel from Carolina Blood and Cancer Care Associates. Patel and his colleagues have seen a 20% to 30% increase in new patients, multiple patients with several different cancers, couples and siblings developing cancers within months of each other, and patients relapsing after years of remission.1
The Mystery of Growing Early-onset Cancer Rates
A growing body of research is confirming that rates of early-onset cancers (those affecting patients younger than 50 years of age) have been rising since the 1990s. Studies have shown increasing rates of early-onset: breast, lung, gastrointestinal, gynecologic, genitourinary, and hematologic cancers, among others.
A 2023 study2 in JAMA Network Open examined patterns in early-onset cancer incidence in the US from 2010-2019. The authors identified over 560,000 patients with early-onset cancer during this period.
Results showed an increase in early-onset cancer incidence from 99.96 cases per 100,000 people in 2010, compared to 102.97 cases per 100,000 in 2019, an annual percentage change (APC) of 0.28% (P =.01).
Meanwhile, cancer incidence for those 50 and older actually decreased (APC, -0.87%; P <.001).
“Overall trends show many of the cancers with the largest increases in early-onset disease are in the gastrointestinal tract, including cholangiocarcinoma, colorectal, gastric, appendix, and pancreatic cancers,” said Dr. Suneel Kamath of the Cleveland Clinic Taussig Cancer Institute.
Mortality rates have also increased by 1% per year according to a 2024 American Cancer Society report.3
Younger patients aged 20-49 diagnosed with colorectal cancer often have larger, faster-growing tumors that have already metastasized when compared to older adults.
The increasing rates of early-onset colorectal cancer “are not due to an increase in germline-syndrome related cancers, as 70% of these cancers are sporadic in younger patients, so there is clearly some environmental exposure(s) that may be a driver,” Dr. Kamath noted.
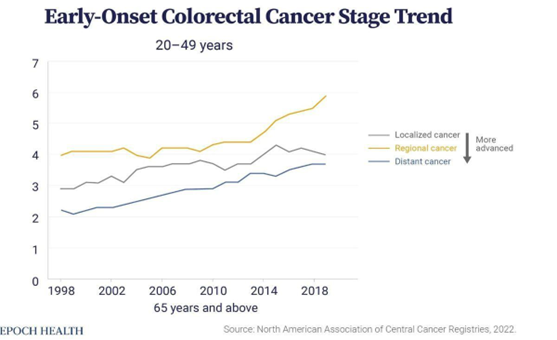
The increasing incidence of early-onset cancers is expected to continue in the near future, said Dr. William M. Grady of Fred Hutchinson Cancer Center.
Researchers suggest that lifestyle changes and reducing exposure to environmental carcinogens could help lower early-onset cancer rates. Implementing cancer screening at younger ages and modifying treatment approaches could improve outcomes for patients who develop early-onset cancers.4
Another 2023 study5 in BMJ Oncology found a 79.1% increase in global early-onset cancer incidence and a 27.7% increase in related deaths between 1990 and 2019.
A third recent study,6 published in Lancet Public Health in July 2024, found that Generation X and millennials in the US have a higher risk of developing 17 cancers compared to older generations.
For certain cancers, people born in 1990 face 2-3 times the risk of those born in 1955.
Types of Cancers That Are Increasing the Most
American Cancer Society (ACS) researchers assessed rates of 34 different cancers among those born between 1920 and 1990, based on diagnoses and deaths from 2000 to 2019.
On average, rates of 17 cancer types have risen with each new generation since 1920. Previous ACS research showed that rates of 11 cancers had been increasing among young adults. The new study added eight more types:
- Gastric cardia cancer (stomach lining)
- Small-intestinal cancer
- Estrogen-receptor-positive breast cancer
- Ovarian cancer
- Liver and bile-duct cancer
- Non-HPV-associated oral and pharynx cancer (women only)
- Anal cancer (men only)
- Kaposi sarcoma (men only)
Rates doubled or tripled for some cancers, including kidney, pancreatic, and small intestinal cancers in both genders. For women, liver cancer incidence has increased 2-3 times since the 1920s. Even cancers that seemed to be declining for baby boomers and older generations are now a greater risk for millennials and Gen X-ers.
More young people are dying from some of these cancers as well; mortality from colorectal, gallbladder, testicular, and uterine cancers has increased over the generations, as has the fatality rate of liver cancer in women.
In another new study,7 researchers from Brigham and Women’s Hospital found that more people under 50 are being diagnosed with cancers of the breast, colon, esophagus, kidney, liver, pancreas, and more — globally — in a trend that sharply increased around 1990.
“From our data, we observed something called the birth cohort effect. This effect shows that each successive group of people born at a later time (e.g. a decade-later) have a higher risk of developing cancer later in life, likely due to risk factors they were exposed to at a young age,” said Dr. Shuji Ogino, professor of pathology and physician-scientist at Brigham and Women’s.
“We found that this risk is increasing with each generation,” Ogino continued. “For instance, people born in 1960 experienced higher cancer risk before they turn 50 than people born in 1950, and we predict that this risk level will continue to climb in successive generations.”
Breast Cancer Incidence Is Rising, Especially In Younger Women
Breast cancer incidence has been on the rise, particularly among White women under the age of 50. The American Cancer Society (ACS)8 which underscores the persistence of racial and ethnic disparities in breast cancer incidence and outcomes, noted an overall 1% annual increase in breast cancer incidence from 2012 to 2021. The additional cases were largely composed of localized-stage and hormone-receptor-positive (HR+) disease, which generally have a better prognosis than more advanced and HR–negative disease.
Although the overall annual increase in breast cancer incidence from 2012 to 2021 was 1%, the increase was steeper among women under age 50, at 1.4% annually vs 0.7% among those aged 50 or older.
The increased incidence of breast cancer in younger women “is an area of concern and an area where we really need to spend more effort trying to understand why,” said lead study author and breast surgeon Adetunji T. Toriola, MD, PhD, MPH, of Washington University in St Louis.9
What Is Causing This Sudden Rise In Cancer?
Several factors may be driving the increase in early-onset cancers, Dr. Grady noted. These include:
“increasing rates of obesity, decreasing physical activity, increasing incidence and younger age of onset of adult-onset diabetes mellitus, increased exposure to chemicals in plastics and microplastics, poor sleep hygiene, and dietary changes such as increased consumption of ultra-processed foods and refined sugars and decreased consumption of whole foods.” 10,11
In the BMJ Oncology study,12 researchers identified dietary factors such as a diet high in red meat and sodium and low in fruit, whole grains, and calcium, as well as alcohol and tobacco abuse as the main risk factors for early-onset cancer.
Researchers from Ohio State University found signs that a high-fat, low-fiber diet may increase inflammation in the gut that prevents it from naturally suppressing tumors. The cells of young people with colorectal cancer also appeared to have aged more quickly, by 15 years on average, than a person’s actual age. That’s unusual, because older people with colorectal cancer don’t have this same increase in cellular aging.13
Recent research has linked a bacteria called Fusobacterium nucleatum to colorectal and other cancers. It’s not unusual for fusobacterium to be present in a person’s mouth, but it is more likely to be found in the intestines of colorectal cancer patients, compared to healthy people. One study found that people with colorectal cancer were five times more likely to have fusobacterium in their stool compared to healthy people.14
One Factor May Be the Overuse Of Antibiotics
Mounting evidence suggests that microbial dysbiosis caused by antibiotic use is a risk factor for several cancers, including colorectal cancer, as well as breast cancer.
“The findings suggest that individuals with genetic risk factors (i.e., family history of CRC) who have experienced early-life antibiotic use on a long-term basis are probably at increased early-onset CRC risk,” the authors concluded. “Given that antibiotics remain valuable in the management of bacterial infections during early life, investigating the pros and cons of early-life antibiotic use is of great significance.”15
Some data has linked long-term or recurrent antibiotic use in early life to an increased risk of early-onset colorectal cancer via alterations in the gut microbiome.16
Triple-negative breast cancer patients who used antibiotics within three years of diagnosis have an increased risk of death, according to a study.17 The gut microbiome is a likely link.
A 2019 systematic review and meta-analysis of observational studies18 that assessed the association between antibiotic use and cancer found that prolonged use of antibiotics is associated with a small increased risk of various cancers throughout a lifetime.
Antibiotics not only act on bacteria that cause infections but also affect the resident healthy microbiota. The observation that the gut microbiome can be permanently perturbed even by short-term or low-dose antibiotic treatment, and that this change can have long-term effects on health, cautions against widespread and potentially unnecessary use of antibiotics, particularly in young children and pregnant women, and illustrates that antibiotics should not be considered harmless.19
Obese individuals exhibit marked differences in the composition of the intestinal microbial community compared to lean subjects. These changes in the gut microbiota precede the clinical manifestation of obesity. Antibiotic-induced changes in the gut microbiota influence host metabolism and lead to fat accumulation.
The damage caused to the intestinal microbiome by antibiotic exposure in the perinatal period appears to program the host to an obesity-prone metabolic type, which persists after the antibiotics have been discontinued and the gut microbiota has recovered.20
Antibiotics up to 6 months before immune checkpoint inhibitor therapy reduced overall survival and progression-free survival across multiple malignancies, according to a study presented at the Virtual 35th Annual Meeting of the Society of Immunotherapy of Cancer.21
There is also a relationship between antibiotics and chemotherapy efficiency. Antibiotic administration appears to be associated with reduced efficacy of neoadjuvant therapy and poor prognosis in breast cancer patients.22
Antibiotics, which were previously believed not to negatively impact the immune system, have now been associated with major dysregulation of the innate immune defense system.23
Obesity and Increased Leptin Levels
The overall increase in obesity is one factor that helps to account for many early-onset cancers. According to a recent study published in JAMA Oncology24 that involved 85,000 women, being overweight (BMI of 25–30) increases the risk of early-onset colorectal cancer by 37%, while obesity (BMI over 30) increases the risk by 93%, almost doubling it.
Dr. Kortmansky explained that obesity alters hormone levels, and that the hormone leptin promotes tumor growth25,26 and is a potent driver of cancer stem cells.27,28 This, in turn, triggers the early onset of other cancers, such as breast, pancreatic, and endometrial cancers. Many studies have demonstrated that elevated chronic serum concentrations of leptin, frequently seen in obese subjects, represent a stimulatory signal for tumor growth.29

NILCO is an acronym that stands for: Notch, IL-1, and leptin crosstalk outcome. It is a complex signaling pathway that plays a role in cancer-cell growth and angiogenesis. Increased leptin binds to receptors on cancer cells and this leads to a cascade that Increases proliferation, Invasion, migration, angiogenesis, and blocks apoptosis.
Obesity Trends in Young Adults
A comprehensive analysis of U.S. obesity trends revealed concerning patterns and projections.
Key Findings from a 2021 study include:
- The U.S. had 15.1 million children and younger teens that were obese. (5-14 years)
- 21.4 million older adolescents in the U.S. were obese. (15-24 years)
Historical Trends from 1990-2021 include:
- Obesity increased more rapidly than overweight.
- In male teens there was a 158.4% increase in obesity rates.
- In female teenagers there was a 185.9% increase in obesity rates. 30
These sobering obesity statistics correlate with the increasing cancer rates in younger adults. Since obesity is a known risk factor for all cancers, the escalating rates of cancer in this younger age bracket begins to make sense.
Natural Compounds That Can Suppress Tumor Growth By Lowering Leptin Levels
Isothiocyanates- are compounds in cruciferous vegetables such as: broccoli, cauliflower, cabbage, and brussels sprouts. They can inhibit the oncogenic actions of leptin in human breast cancer cells by suppressing activation of signal transducer and activator of transcription 3.31
Honokiol- is an active compound in Magnolia bark (Magnolia grandiflora) extract that has been shown to override leptin-induced tumor progression. Honokiol significantly inhibits leptin-induced breast-cancer-cell growth, invasion, migration, and leptin-induced breast-tumor-xenograft growth.32
Vitamin D- suppresses leptin stimulation of cancer growth through microRNA. hTERT (human telomerase reverse transcriptase) downregulation is a key event mediating the anti-leptin activity of vitamin D in estrogen-sensitive tumors in women.33
Conjugated linoleic acid (CLA)- has been shown to decrease fat and increase lean mass in several animal studies. CLA is found predominantly in “ORGANIC” milk fat from free-range grass-fed cows. CLA promotes weight loss while retaining lean muscle mass. It appears to reduce body fat, improve insulin sensitivity, and lower leptin levels.34,35
In a study on the Chinese population, people who took CLA supplements for 12 weeks lost more body fat and inches off their waistlines than those who didn’t take the supplements. 36
Reggiano Parmesan Cheese– is one of my favorite foods in the world and is very rich in CLA.37 The Reggiano title refers to the region in Italy where this cheese originates. The health benefits of nutrient-dense parmesan cheese include nourishing the immune system, digestive system, nervous system, and bone health (it contains over 1000 mg of calcium per 100 grams of cheese). Reggiano Parmesan is easily digested, contains ready-to-use proteins and lipids, is lactose free, rich in calcium, and has possible prebiotic and probiotic effects.38
The probiotic Lactobacillus rhamnosus (LR) contained in Parmigiano Reggiano plays a crucial role in producing high levels of enzymes responsible for proteolytic activity, fatty-acid metabolism, and for the release of butyrate. LR has been very well studied, and has been shown in a number of different settings to aid in immunity. Various health effects are well documented including the prevention and treatment of gastrointestinal infections, diarrhea, stimulation of immune responses, and prevention of certain allergic symptoms.39 There is good
evidence for the use of LR in the prevention and treatment of diarrhea from acute infections or antibiotic use.40 One study found that LR could be just as helpful as an antibiotic for treating urinary tract infections.41
Seaweed– Dietary intake of seaweeds is associated with a lower prevalence of chronic disease. Seaweeds have high-nutritive value due to their rich content of: fiber, polyunsaturated fatty acids, minerals, and other bioactive compounds.42 These compounds include peptides, polyphenols (phlorotannins and bromophenols), polysaccharides (alginate, fucoidans, laminarin), carotenoids (fucoxanthin), and phytosterols, abundant in Undaria pinnatifida (Sea Mustard, Miyeok, Wakame), Saccharina japonica (formerly Laminaria japonica; Kelp, Dasima, Kombu), and Saccharina latissima (Sugar kelp). Although seaweed products show promising preventive and therapeutic effects, it is also crucial to consider potential health risks such as heavy metal toxicity from polluted waters.43
Recently, many studies have presented the anti-obesity effects of brown seaweeds and their secondary metabolites, such as phlorotannins. However, studies that target the appetite hormone leptin are few. In one study, researchers explored the natural anti-obesity agents from brown seaweeds targeting leptin, including ishophloroglucin A, found in Ishige okamurae (Yendo) from Korea.
“Ishophloroglucin A favorably docks to the leptin receptor. Ishophloroglucin A reduced body weight and food intake in mice. Leptin signaling in the hypothalamus and insulin signaling in peripheral organs were stimulated.” 44
Could COVID-19 Vaccines Be a Contributor?
There is a lot of speculation around the S Protein from both the COVID-19 vaccines, as well as chronic COVID-19, which involves an ongoing state of inflammation and immune weakness and vulnerability.
In a review published on Dec. 26, 2023,45 the authors of a study found that non-live vaccines tend to increase a person’s risk of all-cause mortality. The researchers found that non-live vaccines such as influenza, COVID-19, hepatitis B, and diphtheria-tetanus-pertussis tend to cause adverse nonspecific effects (NSEs), increasing a person’s risk of all-cause mortality and infections from other diseases.
In the case of COVID-19 vaccines, live vaccines were likely not considered due to concerns about the formation of recombinant viruses when a vaccinated person comes into contact with the current circulating viral strain.46
The COVID-19 vaccines may be associated with adverse events, increased inflammation, and immunological stress, because of the presence of highly-toxic spike proteins which studies now link to long COVID and vaccine injuries.47
How Might the microRNA Vaccines Disrupt Cellular Function?
According to a paper from June, 2022 in Food Chem Toxicol,48 “Immune cells that have taken up the vaccine nanoparticles release into circulation large numbers of exosomes containing spike protein along with critical microRNAs that induce a signaling response in recipient cells at distant sites.”
The researchers also found disturbances in protein synthesis and cancer surveillance that have a potential link to:
- Neurodegenerative disease
- Myocarditis
- Immune thrombocytopenia
- Bell’s Palsy
- Liver disease
- Impaired adaptive immunity
- Impaired DNA damage response
- Tumorigenesis
However, the same could be said about the virus itself, especially in those with long COVID.
For me, the argument is less about the vaccine risk-to-benefit ratio, and more about the crime of not sharing the very good news and overwhelming data on the beneficial effects of herbal, nutritional, and dietary medicine for these infections.
These approaches have clearly demonstrated their ability to reduce the onset of COVID-19, its duration, severity, hospitalization rates, duration of hospital stays, and deaths from COVID-19. This is the real crime. Because of this proven corruption, I cannot believe or trust anything that the establishment tells us to be true and is in our best health interests.
Cancer Rates and COVID-19 Vaccines in Japan
During the COVID-19 pandemic, excess deaths including cancer have become a concern in Japan, which has a rapidly aging population. This study49 aimed to evaluate how age-adjusted mortality rates (AMRs) for different types of cancer in Japan changed during the COVID-19 pandemic (2020-2022). Official statistics from Japan were used to compare observed annual and monthly death rates with predicted rates based on pre-pandemic (2010-2019) figures using logistic regression analysis. No significant excess mortality was observed during the first year of the pandemic (2020).
However, some excess cancer mortalities were observed in 2021 after mass vaccination with the first and second vaccine doses, and significant excess mortalities were observed for all cancers and some specific types of cancer (including ovarian, leukemia, prostate, lip/oral/pharyngeal, pancreatic, and breast) after mass vaccination with the third dose in 2022. Mortality rates for the four cancers with the most deaths (lung, colorectal, stomach, and liver) showed a decreasing trend until the first year of the pandemic in 2020, but the rate of decrease slowed in 2021 and 2022.
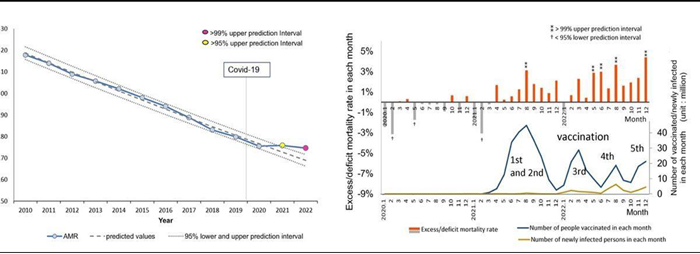
Age-adjusted mortality rates (AMRs) over time and excess mortality in each month: all cancers.
Diet & Lifestyle Factors That Make A Difference
Almost 50% of the total global disease burden is estimated to be associated with modifiable risk factors.50 Why isn’t there more of a movement to improve people’s lives through health optimization utilizing diet, lifestyle, herbal, and nutritional modalities to improve everyone’s lives and reduce the risk of cancer and other chronic diseases?
For example, a recent meta-analysis found that a Mediterranean diet reduced cardiovascular disease and mortality in diabetes.
Another recent study, which included over 40,000 participants, found that a diet rich in fruits, vegetables, and legumes was associated with a significantly reduced risk of all-cause, cardiovascular, and cancer mortality.51
The Mederi-Care Approach Can Reduce Cancer Risk
At the foundation of the Mederi-Care approach to health and healing is a focus on nourishing the root system where we enhance and optimize robustness, auto-regulation, and auto-organization at the molecular, cellular, and organ-system levels. Mederi-Care utilizes a body of practices, procedures, and systems that together, form the Mederi-Care methodology. Through the educated use of these timeless principles, we can begin to turn the tide of these earlier-onset cancers.
While the increased cancer rates among the younger population are complex and multifactorial, we know that nutrition, obesity, sleep, environmental toxins, antibiotics, vaccines, and stress can all play a role. These are all factors that can be shifted through: education, connection to nature, persistence, care, and love.
Let’s embrace the principle of prevention by educating ourselves and others about these risk factors that are leading younger generations to be less healthy than the preceding ones.
Let’s promote natural health and detoxification practices that can decrease these risks, and always remember that love should be at the root of all that we do.
About the Author:
Donald R. Yance is the founder of the Mederi Center. A Clinical Master Herbalist and Certified Nutritionist, Donnie is renowned for his extraordinary knowledge and deep understanding of the healing properties of plants and nutrition, as well as of epigenetics, laboratory medicine, oncologic pathology, and molecular oncology. He is a professional member of the American Herbalists Guild, National Association of Nutrition Professionals, Academy of Integrative Health and Medicine, and the Society for Integrative Oncology.
References:
1 Could COVID-19 be behind the rise in rare and aggressive cancers?, July 1st, 2024, https://www.advisory.com/daily-briefing/2024/06/12/covid-cancer
2 Koh B, Tan DJH, Ng CH, et al. Patterns in Cancer Incidence Among People Younger Than 50 Years in the US, 2010 to 2019. JAMA Netw Open. 2023;6(8):e2328171. doi:10.1001/jamanetworkopen.2023.28171
3 https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2024/2024-cancer-facts-and-figures-acs.pdf
4 Tori Rodriguez, MA, LPC, AHC, January 12, 2024, Early-Onset Cancers Are on the Rise: Why It’s Happening and What We Can Do, https://www.cancertherapyadvisor.com/home/cancer-topics/general-oncology/early-onset-cancers-on-the-rise-why-what-we-can
5 Zhao J, Xu L, Sun J, et al. Global trends in incidence, death, burden and risk factors of early-onset cancer from 1990 to 2019. BMJ Oncol. Published online September 5, 2023. doi:10.1136/bmjonc-2023-000049
6 Hung, Sung et. al., Generation X, millennials in US have higher risk of developing 17 cancers compared to older generations (2024, July 31) retrieved 1 August 2024 from https://medicalxpress.com/news/2024-07-generation-millennials-higher-cancers-older.html
7 Ugai T et al. “Is early-onset cancer an emerging global epidemic? Current evidence and future implications.” Nature Reviews Clinical Oncology DOI: 10.1038/s41571-022-00672-8
8 https://acsjournals.onlinelibrary.wiley.com/doi/full/10.3322/caac.21863
9 Xu S, Murtagh S, Han Y, Wan F, Toriola AT. Breast Cancer Incidence Among US Women Aged 20 to 49 Years by Race, Stage, and Hormone Receptor Status. JAMA Netw Open. 2024;7(1):e2353331. doi:10.1001/jamanetworkopen.2023.53331
10 Ugai T et al. “Is early-onset cancer an emerging global epidemic? Current evidence and future implications.” Nature Reviews Clinical Oncology DOI: 10.1038/s41571-022-00672-8
11 Sayed A, Abramov D, Fonarow GC, et al. Reversals in the Decline of Heart Failure Mortality in the US, 1999 to 2021. JAMA Cardiol. Published online April 24, 2024. doi:10.1001/jamacardio.2024.0615
12 h Zhao J, Xu L, Sun J, et al. Global trends in incidence, death, burden and risk factors of early-onset cancer from 1990 to 2019. BMJ Oncol. Published online September 5, 2023. doi:10.1136/bmjonc-2023-000049
13 Young People’s Gut Bacteria May Drive Colorectal Cancer Risk - Medscape - June 06, 2024.
14 Reynolds, Sharon, Scientists Link a Single Type of Bacteria to Colorectal Cancer, April 19, 2024, NIH, https://www.cancer.gov/news-events/cancer-currents-blog/2024/colorectal-cancerfnac2bacteria#:~:text=Over%20the%20last%20decade%2C%20one,contributor%20to%20colorectal%20cancer%20growth.
15 Fangyuan Jiang, Daniel Boakye, Jing Sun, Lijuan Wang, Lili Yu, Xuan Zhou, Jianhui Zhao, Zilong Bian, Peige Song, Yazhou He, Yingshuang Zhu, Jie Chen, Shuai Yuan, Mingyang Song, et.al., Association between antibiotic use during early life and early-onset colorectal cancer risk overall and according to polygenic risk and FUT2 genotypes, July 28, 2023, International Journal of Cancer, https://doi.org/10.1002/ijc.34648
16 McDowell, R., Perrott, S., Murchie, P. et al. Oral antibiotic use and early-onset colorectal cancer: findings from a case-control study using a national clinical database. Br J Cancer 126, 957–967 (2022). https://doi.org/10.1038/s41416-021-01665-7
17 Ransohoff, J.D., Ritter, V., Purington, N. et al. Antimicrobial exposure is associated with decreased survival in triple-negative breast cancer. Nat Commun 14, 2053 (2023). https://doi.org/10.1038/s41467-023-37636-0
18 Petrelli F, Ghidini M, Ghidini A, Perego G, Cabiddu M, Khakoo S, Oggionni E, Abeni C, Hahne JC, Tomasello G, Zaniboni A. Use of Antibiotics and Risk of Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Cancers (Basel). 2019 Aug 14;11(8):1174. doi: 10.3390/cancers11081174.
19 Willing, C. P. et al. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat. Rev. Microbiol. 9, 233–243 (2011).
20 Turta and Rautava, Antibiotics, obesity and the link to microbes – what are we doing to our children? BMC Medicine (2016) 14:57 DOI 10.1186/s12916-016-0605-7
21 Ramos, Eric November 10, 2020, DG News, Presentation title: Antibiotic Administration Prior to Immunotherapy Leads to Poor Overall Survival Across Multiple Malignancies. Abstract 774, https://dgnews.docguide.com/antibiotic-administration-prior-immunotherapy-leads-poor-overall-survival-across-multiple?overlay=2&nl_ref=newsletter&pk_campaign=newsletter&nl_eventid=25521&nl_campaignid=1607&MemberID=303049504
22 Zhang X, Yu L, Shi J, Li S, Yang S, Gao W, Yang S, Cheng M, Wang H, Guo Z, Geng C. Antibiotics modulate neoadjuvant therapy efficiency in patients with breast cancer: a pilot analysis. Sci Rep. 2021 Jul 7;11(1):14024. doi: 10.1038/s41598-021-93428-w.
23 Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010 Feb;16(2):228-31. doi: 10.1038/nm.2087. Epub 2010 Jan 17.
24 Liu PH, Wu K, Ng K, Zauber AG, Nguyen LH, Song M, He X, Fuchs CS, Ogino S, Willett WC, Chan AT, Giovannucci EL, Cao Y. Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol. 2019 Jan 1;5(1):37-44. doi: 10.1001/jamaoncol.2018.4280. Erratum in: JAMA Oncol. 2019 Apr 1;5(4):579. doi: 10.1001/jamaoncol.2019.0286. PMID: 30326010; PMCID: PMC6382547.
25 Sultana R, Kataki AC, Borthakur BB, Basumatary TK, Bose S. Imbalance in leptin-adiponectin levels and leptin receptor expression as chief contributors to triple negative breast cancer progression in `Northeast India. Gene. 2017 Jul 20;621:51-58. doi: 10.1016/j.gene.2017.04.021. Epub 2017 Apr 14.
26 Angelucci A, Clementi L, Alesse E. Leptin in Tumor Microenvironment. Adv Exp Med Biol. 2020;1259:89-112. doi:10.1007/978-3-030-43093-1_6
27 Lipsey CC, Harbuzariu A, Robey RW, Huff LM, Gottesman MM, Gonzalez-Perez RR. Leptin Signaling Affects Survival and Chemoresistance of Estrogen Receptor Negative Breast Cancer. Int J Mol Sci. 2020 May 27;21(11):3794. doi: 10.3390/ijms21113794.
28 Olea-Flores M, Juárez-Cruz JC, Zuñiga-Eulogio MD, Acosta E, García-Rodríguez E, Zacapala-Gomez AE, Mendoza-Catalán MA, Ortiz-Ortiz J, Ortuño-Pineda C, Navarro-Tito N. New Actors Driving the Epithelial-Mesenchymal Transition in Cancer: The Role of Leptin. Biomolecules. 2020 Dec 15;10(12):1676. doi: 10.3390/biom10121676.
29 Olea-Flores M, Juárez-Cruz JC, Zuñiga-Eulogio MD, Acosta E, García-Rodríguez E, Zacapala-Gomez AE, Mendoza-Catalán MA, Ortiz-Ortiz J, Ortuño-Pineda C, Navarro-Tito N. New Actors Driving the Epithelial-Mesenchymal Transition in Cancer: The Role of Leptin. Biomolecules. 2020 Dec 15;10(12):1676. doi: 10.3390/biom10121676.
30 Ng, Marie et al. National-level and state-level prevalence of overweight and obesity among children, adolescents, and adults in the USA, 1990–2021, and forecasts up to 2050, The Lancet, Volume 0, Issue 0, Online first November 14, 2024, Open access,
31 Kim SH, Nagalingam A, Saxena NK, Singh SV, Sharma D. Benzyl isothiocyanate inhibits oncogenic actions of leptin in human breast cancer cells by suppressing activation of signal transducer and activator of transcription 3. Carcinogenesis. 2011 Mar;32(3):359-67. doi: 10.1093/carcin/bgq267. Epub 2010 Dec 16. PMID: 21163886; PMCID: PMC3105585.
32 Avtanski DB, Nagalingam A, Kuppusamy P, Bonner MY, Arbiser JL, Saxena NK, Sharma D. Honokiol abrogates leptin-induced tumor progression by inhibiting Wnt1-MTA1-β-catenin signaling axis in a microRNA-34a dependent manner. Oncotarget. 2015 Jun 30;6(18):16396-410. doi: 10.18632/oncotarget.3844.
33 Kasiappan R1, Sun Y1, Lungchukiet P1, Quarni W1, Zhang X2, Bai W3. Vitamin D suppresses leptin stimulation of cancer growth through microRNA, Cancer Res. 2014 Nov 1;74(21):6194-204. doi: 10.1158/0008-5472.CAN-14-1702. Epub 2014 Sep 24.
34 Corl BA, Mathews Oliver SA, Lin X, Oliver WT, Ma Y, Harrell RJ, Odle J. Conjugated linoleic acid reduces body fat accretion and lipogenic gene expression in neonatal pigs fed low- or high-fat formulas. J Nutr. 2008 Mar;138(3):449-54.
35 Esmaeili Shahmirzadi F, Ghavamzadeh S, Zamani T. The Effect of Conjugated Linoleic Acid Supplementation on Body Composition, Serum Insulin and Leptin in Obese Adults. Arch Iran Med. 2019 May 1;22(5):255-261. PMID: 31256599.
36 Chen SC, Lin YH, Huang HP, Hsu WL, Houng JY, Huang CK. Effect of conjugated linoleic acid supplementation on weight loss and body fat composition in a Chinese population. Nutrition. 2012 May;28(5):559-65. doi: 10.1016/j.nut.2011.09.008. Epub 2012 Jan 20. PMID: 22261578.
37 FORMIGONI, ANDREA;BROGNA, NICO;MERENDI, FLAVIA;MORDENTI, ATTILIO;BIAGI, GIACOMO, Concentration of conjugated linoleic acid (CLA) and other fatty acids in Parmigiano-reggiano cheese, January 2008, pp. 306-306. (Intervento presentato al convegno XXV Jubilee World Buiatrics Congress tenutosi a Budapest, Hungary nel 6-11.07.2008).
38 Castellone V, Bancalari E, Rubert J, Gatti M, Neviani E, Bottari B. Eating Fermented: Health Benefits of LAB-Fermented Foods. Foods. 2021 Oct 31;10(11):2639. doi: 10.3390/foods10112639. PMID: 34828920; PMCID: PMC8620815.
39 Lazar V, Ditu LM, Pircalabioru GG, Gheorghe I, Curutiu C, Holban AM, Picu A, Petcu L, Chifiriuc MC. Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front Immunol. 2018 Aug 15;9:1830. doi: 10.3389/fimmu.2018.01830.
40 Lazar V, Ditu LM, Pircalabioru GG, Gheorghe I, Curutiu C, Holban AM, Picu A, Petcu L, Chifiriuc MC. Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front Immunol. 2018 Aug 15;9:1830. doi: 10.3389/fimmu.2018.01830.
41 Malczewski AB, Ketheesan N, Coward JIG, Navarro S. Enhancing Checkpoint Inhibitor Therapy in Solid Tissue Cancers: The Role of Diet, the Microbiome & Microbiome-Derived Metabolites. Front Immunol. 2021 Jul 7;12:624434. doi: 10.3389/fimmu.2021.624434. PMID: 34305883; PMCID: PMC8293987.
42 Bermano G, Stoyanova T, Hennequart F, Wainwright CL. Seaweed-derived bioactives as potential energy regulators in obesity and type 2 diabetes. Adv Pharmacol. 2020;87:205-256. doi: 10.1016/bs.apha.2019.10.002. Epub 2019 Dec 3. PMID: 32089234.
43 Hyungryun Jang, Jaeeun Lee, Young-Ki Park, Ji-Young Lee, Exploring the health benefits and concerns of brown seaweed consumption: A comprehensive review of bioactive compounds in brown seaweed and its potential therapeutic effects, Journal of Agriculture and Food Research, Volume 17, 2024, 101215, ISSN 2666-1543, https://doi.org/10.1016/j.jafr.2024.101215.
44 Nalae Kang, Seyeon Oh, Seo-Young Kim, Hyosang Ahn, Myeongjoo Son, Soo-Jin Heo, Kyunghee Byun, You-Jin Jeon, Anti-obesity effects of Ishophloroglucin A from the brown seaweed Ishige okamurae (Yendo) via regulation of leptin signal in ob/ob mice, Algal Research, Volume 61, 2022, 102533, ISSN 2211-9264, https://doi.org/10.1016/j.algal.2021.102533.
45 Rubio-Casillas A, Rodriguez-Quintero CM, Redwan EM, Gupta MN, Uversky VN, Raszek M. Do vaccines increase or decrease susceptibility to diseases other than those they protect against? Vaccine. 2023 Dec 28:S0264-410X(23)01506-2. doi: 10.1016/j.vaccine.2023.12.060. Epub ahead of print. PMID: 38158298.
46 Adler JM, Martin Vidal R, Voß A, Kunder S, Nascimento M, Abdelgawad A, Langner C, Vladimirova D, Osterrieder N, Gruber AD, Kunec D, Trimpert J. A non-transmissible live attenuated SARS-CoV-2 vaccine. Mol Ther. 2023 Aug 2;31(8):2391-2407. doi: 10.1016/j.ymthe.2023.05.004. Epub 2023 May 26. PMID: 37263272; PMCID: PMC10214529.
47 Cosentino M, Marino F. The spike hypothesis in vaccine-induced adverse effects: questions and answers. Trends Mol Med. 2022 Oct;28(10):797-799. doi: 10.1016/j.molmed.2022.07.009. Epub 2022 Sep 12. PMID: 36114089; PMCID: PMC9494717.
48 Seneff S, Nigh G, Kyriakopoulos AM, McCullough PA. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem Toxicol. 2022 Jun;164:113008. doi: 10.1016/j.fct.2022.113008. Epub 2022 Apr 15.
49 Gibo M, Kojima S, Fujisawa A, et al. (April 08, 2024) Increased Age-Adjusted Cancer Mortality After the Third mRNA-Lipid Nanoparticle Vaccine Dose During the COVID-19 Pandemic in Japan. Cureus 16(4): e57860. doi:10.7759/cureus.57860
50 Assessing the evidence of risk. Nat Med 28, 1967 (2022). https://doi.org/10.1038/s41591-022-02039-z
51 Liu W, Hu B, Dehghan M, Mente A, Wang C, Yan R, Rangarajan S, Tse LA, Yusuf S, Liu X, Wang Y, Qiang D, Hu L, Han A, Tang X, Liu L, Li W; PURE-China Investigators. Fruit, vegetable, and legume intake and the risk of all-cause, cardiovascular, and cancer mortality: A prospective study. Clin Nutr. 2021 Jan 27:S0261-5614(21)00026-1. doi: 10.1016/j.clnu.2021.01.016. Epub ahead of print. PMID: 33581953.


Thanks for compiling all this research and especially for the comment
“The real crime is not promoting effective and safe herbs.”
They work for me every time and I have Gaia to thank!
Thanks for all the research especially the comment
The real crime was not promoting effective safe and effective herbs.
They work for me all the time and I can simply thank Gaia.
Thank you, Donnie for this fascinating well-documented article. I especially appreciate your pointing out that there is much we can do to thwart disease and create heath.
Another great post. Thanks, Dr Yance! I hope that RFK and the new administration follows your lead!
Thanks Donnie for another balanced report. So good to have some real data