Gankyrin and Cancer
A better understanding of Gankyrin, glutamine, and glutamate production is crucial for cancer patients and their doctors.
Cancer develops when cells start growing too fast. This process kicks off when genes responsible for regular cell growth transform into cancer genes, also known as oncogenes. The term “oncogene” is derived from the Greek words for tumor (“onco”) and “gignere,” which means to create, generate, or induce. While normal genes can be turned off, oncogenes cannot.
Rapidly proliferating cancer cells absorb glutamine from plasma through various amino acid transporters. This glutamine is then converted to glutamate within the mitochondria. Two forms of glutaminase drive this process: kidney-type glutaminase (GLS1) and liver-type (GLS2).
GLS1 is overexpressed in various cancer cells, and this phenotype is associated with advanced disease stages and a poorer prognosis.[1]
To fuel rapid cell growth, the oncogenes Gankyrin/c-myc reprogram cancer cell metabolism in a substantially different way from normal cells.
C-myc-induced metabolic signature is characterized by enhanced glucose and glutamine uptake, increased lactate production, and altered amino acid metabolism.[2]
What is Gankyrin?
Gankyrin is a crucial oncoprotein. An oncoprotein is a protein involved in regulating or synthesizing proteins linked to the growth of cancerous tumors.
As researchers in California explain in a 2020 article published in Nature Reviews Cancer, human oncoproteins help transform normal cells into cancer cells by dysregulating signaling pathways involved in cell growth.
Gankyrin appears to be a reliable biomarker for cancer metastasis and prognosis, according to a 2021 peer-reviewed article.
Gankyrin Inhibits Tumor Suppression Proteins
Gankyrin plays a significant role in tumorigenicity and metastasis of liver cancer, known as hepatocellular carcinoma (HCC). Cancer of the liver, which is becoming more common, causes over 12,000 deaths a year in the United States alone and more than 700,000 yearly deaths worldwide. Symptoms of liver cancer include unexplained weight loss, stomach discomfort, yellowing of the skin and the whites of the eyes, nausea or vomiting, itching, as well as fever.
The oncogenic role of gankyrin has been found to stem from the inhibition of two ubiquitous tumor suppressor proteins: retinoblastoma protein (pRb) and P53.
It has also been found to modulate several vital cellular signaling pathways, including Wnt/β-Catenin, NF-κB, STAT3/Akt, IL-1β/IRAK-1, and RhoA/ROCK. [3]
Gankyrin, Glycolysis, and Glutaminolysis
Gankyrin plays a crucial role in both glycolysis and glutaminolysis.
Gankyrin increases glucose consumption, lactate production, glutamine consumption, and glutamate production in liver cancer. It does so by upregulating the expression of the transporters and enzymes involved in energy metabolism, specifically glycolysis and glutaminolysis. Glycolysis is the utilization of glucose for energy, and glutaminolysis is the utilization of glutamine for energy. These transporters include HK2, GLUT1, LDHA, PKM2, ASCT2 and GLS1.
Gankyrin also drives glycolysis and glutaminolysis by upregulating the oncogene c-Myc via activating β-catenin signaling.
Scientists believe that c-Myc-mediated metabolic reprogramming might contribute to tumorigenicity, metastasis (when cancerous cells spread from one part of the body to another), and drug resistance. This all may be induced by Gankyrin.
Stopping Tumors That Have High Gankyrin Levels
Gankyrin functions as an essential regulator in glycolysis and glutaminolysis through activation of the β-catenin/c-Myc to promote tumorigenesis, metastasis, and drug resistance in human liver cancer.[4]
Imagine glutamine as a baseball and c-Myc as the pitcher. When the pitcher aims a pitch at the batter’s head, the batter doesn’t blame the baseball itself and try to catch it. Instead, the batter gets upset with the pitcher and charges toward the pitcher’s mound.
Think of glutamine as the unsung hero in this story. It’s wrongly accused of causing trouble when the real problem lies deeper. In simpler terms, cancer cells have hijacked glutamine to power themselves up.
Sorafenib is a targeted cancer drug. It is used to treat liver cancer as well as thyroid cancer, and, rarely, kidney cancer.
Regorafenib is another anti-cancer drug.
Researchers have found a significant correlation between Gankyrin and β-catenin expression levels in people with liver cancer. This combination is a powerful predictor of poor prognosis.
However, scientists have also found that a c-Myc inhibitor can work with these drugs, Sorafenib or Regorafenib, to suppress tumors that have high Gankyrin levels.
Breaking Down Lactate
Your body usually gets rid of lactate as quickly as it’s produced, especially when everything is running smoothly. However, in times of illness or when your organs aren’t working as they should, lactate can build up in your bloodstream.
Severe dehydration, anemia, leukemia, liver disease, and liver cancer can all prevent the liver from effectively breaking down lactic acid in the blood, which in turn causes lactate to accumulate.
Lactate Promotes Glutamine Uptake and Metabolism in Cancer Cells
Although glycolysis and glutaminolysis can work together to promote cell growth, which is not desired in the case of cancer, it seems that oxidative lactate metabolism acts as a counterforce against glycolysis.
Oxidative cancer cells and their hypoxic/glycolytic counterparts have a mutually beneficial relationship. In simple terms, cancer cells that still rely on some oxygen collaborate with those that have adapted to low-oxygen environments.
Tumors are often a mix of various kinds of cancer cells, including cancer stem cells or CSCs.
Over the past two decades, extensive research has consistently pointed to a specific subgroup of cancer cells with the unique capability to initiate and drive tumorigenesis. These cells have been designated as cancer stem cells or CSCs.
The presence of CSCs within a tumor is frequently associated with increased invasiveness and the tumor’s propensity to metastasize or spread to other parts of the body.[5]
Lactate Signaling Promotes Glutamine Uptake
Intracellular lactate signaling promotes glutamine uptake and metabolism in oxidative cancer cells.
This depends on the uptake of extracellular lactate by monocarboxylate transporter 1 (MCT1).
Lactate first stabilizes hypoxia-inducible factor-2a (HIF-2a), and HIF-2a then transactivates c-Myc in a pathway that mimics a response to hypoxia.
Consequently, lactate-induced c-Myc activation triggers the expression of glutamine transporter ASCT2 and of glutaminase, resulting in improved glutamine uptake and catabolism.
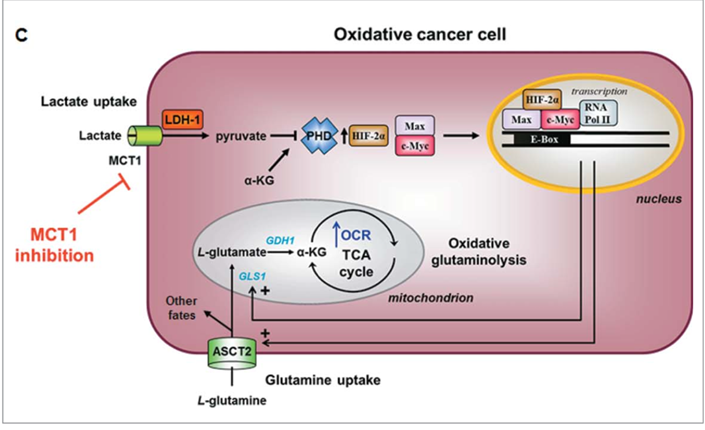
Lactate Stimulates Oxidative Glutaminolysis[6]
Glioblastoma Multiforme
There’s a fast-growing brain cancer called Glioblastoma Multiforme, or GBM. Also called grade IV astrocytoma, this extremely aggressive cancer causes tumors and can invade brain tissue, though it generally does not spread to other parts of the body.
GBM, if left untreated, can cause death in a matter of months.
About 30 people in a million suffer from this kind of cancer.
Like other forms of cancer, it appears to be on the rise worldwide.
SNAT3 and Glutamine
Back in 2004, a group of Polish scientists noticed a substantial increase in the expression of mRNA transcripts of glutamine transporters, specifically system N transporter 3 (SNAT3) and alanine/serine/cysteine-preferring transporter 2 (ASCT2), in samples of Glioblastoma Multiforme (GBM).[7]
Furthermore, a different team of scientists later confirmed that overexpression of ASCT2 contributes to the development of aggressive brain cancers. They conducted their experiments using a GBM-derived C6 cell line.[8]
This research team discovered that within cancerous cells, the excessive uptake of glutamine serves three primary purposes:
- Generating NADPH (nicotinamide adenine dinucleotide phosphate): NADPH is a crucial electron donor that supplies the necessary energy for anabolic reactions and helps maintain a balanced redox state in the cells.
- Boosting the production of glutamate: Glutamate, an important molecule, is significantly increased in production due to the excess glutamine uptake.
- Facilitating the uptake of essential amino acids (EAA): Glutamine uptake helps the cells absorb essential amino acids, which are vital for various cellular processes and functions.
The metabolic reprogramming involving NADPH renders cancer cells reliant on the metabolic network for their antioxidant capacity. However, it also renders these cells more vulnerable to oxidative stress.
So, modulating the unique NADPH homeostasis of cancer cells might be an effective strategy to eliminate these cells.[9]
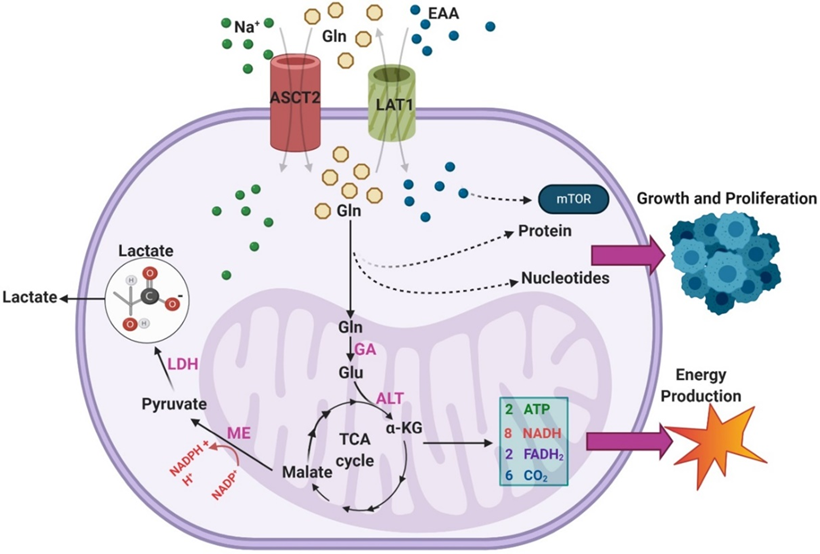
The illustration above shows that glutamine enters the TCA cycle as α-KG through a two-step process involving glutaminase and alanine aminotransferase (ALT).
From there, glutamine is eventually converted to malate and, through malic enzyme (ME) activity, to pyruvate in a reaction that generates NADPH.[10]
This pyruvate is terminally converted to lactate by LDH, and the lactate is excreted.[11]
One study found that over 60% of glutamine was converted to lactate in the SF188 glioblastoma cell line, indicating this pathway is highly active in GBM.
The resultant NADPH provides energy for fatty acid and nucleotide synthesis, which provides a diverse energy source for cancer cells. Cancer cells adapt and continue to capitalize on numerous metabolic pathways for their huge appetite.
Additionally, researchers found that the majority of intracellular oxaloacetic acid (OAA) in the cells came from glutamine rather than glucose, suggesting that glutamine uptake is necessary to provide OAA for continued citrate synthase activity.
This mechanism is particularly important in the genes that code for isocitrate dehydrogenases in gliomas or IDH-mutant GBMs.
It appears that glutamine metabolism replenishes TCA cycle intermediates that would not be present in high concentrations in the absence of cancer.[12]
An overview of the pathways through which exogenous glutamine (Gln) is utilized within GBMs.
Overexpression of ASCT2 in GBMs leads to enhanced Gln uptake so that it can be used as a substrate in the biosynthesis of nucleotides and proteins.
The transport of Gln out of the cell in exchange for excitatory amino acids (EAA) via LAT1 is also upregulated in GBM, leading to enhanced mTOR signaling.
Gln can also feed into the TCA cycle via a two-step process that results in the generation of α-KG.
During the TCA cycle, a series of reactions converts α-KG into malate.
Malate is then further processed to form pyruvate, a reaction that generates NADPH to support growth.[13]
Glutamine May Help Cancer Patients
As I wrote about recently, glutamine is the body’s most abundant free amino acid in the body. Cells use glutamine for both bioenergetic and biosynthetic needs.
When your cells absorb glutamine, it gets transformed into glutamate. This conversion is carried out by an enzyme called mitochondrial glutaminase, which tends to be more upregulated (higher) in cancer cells.[14],[15]
While this suggests limiting glutamine in your diet and supplements, a strategy that appears to make sense from a biochemical perspective, oral glutamine has actually been found to have beneficial effects on overall health, even for cancer patients.
Glutamine May Help Counteract the Negative Effects of Radiation
One important study by a team of oncologists in Greece found that patients suffering from head and neck cancers and acute radiation poisoning showed reduced symptoms when they were administered glutamine.
The research team found that older adults dealing with squamous cell carcinoma of the head and neck, especially those in delicate health who couldn’t tolerate daily radiation and chemotherapy, could potentially receive a safer and more effective treatment by undergoing an alternative radiation schedule along with glutamine supplementation.[16]
Oncogene‐derived metabolic reprogramming is important for the anabolic growth of cancer cells, which is now thought not simply to rely on sugar (glycolysis) but also on fatty acids, various amino acids, and nutrients like iron and copper.
Gankyrin appears to be a major driver of a cancer cell’s ability to fuel cancer cells.[17] All of this means that Gankyrin can be considered a potential candidate for diagnosis and a potential clue for cancer treatment strategies.
Natural Compounds to Target Gankrin/c-myc
Natural compounds are among the current strategies aimed at targeting Gankyrin/c-myc. For example, Vitamin D signaling can suppress the expression of genes regulated by c-myc.[18]
Some of the botanical extracts and natural compounds that I frequently employ in my protocols, that have been found to inhibit cancer via down-regulation of Gankyrin/c-myc include:
- Withaferin A[19] from Ashwagandha (Withania somnifera)
- Oridonin [20],[21] from Rabdosia rubescens
- Artemisinin [22] Artemisia annua
- Parthenolide from Feverfew (Tanacetum parthenium)[23]
- EGCG (Epigallocatechin-3-gallate) from Green Tea (Camellia sinensis) [24]
- Amla (Emblica officinalis)[25]
- Wedelolactone [26] from Eclipta alba
Finally, suppressing LDH (A) activity is an alternative approach to interfere with lactate metabolism. Blocking the excretion of lactate from cancer cells through inhibiting monocarboxylate transporter activity is an indirect yet effective way to reduce glutamine uptake by cancer cells.
Quercetin and potassium bicarbonate are two supplements I recommend to facilitate this.
In my clinic, we view cancer as an interplay between the health of the person, the microenvironment inside the person’s body, the macroenvironment (exposures in the environment at large), and the characteristics of the cancer energy itself. All together, the illness is like a puzzle that we can solve by fitting the various pieces together, small and large alike, biochemical and spiritual, obvious and subtle.
The Mederi Care Model, A New Way Forward
Our philosophy, Mederi Care, challenges the current healthcare model. We don’t fight against cancer.
We find a way forward out of cancer and into better health.
We ask new and different questions that will lead to new and better answers. We’re not in reaction, rebellion, or opposition to the mainstream.
Negative energy quickly becomes another form of righteousness, and we don’t have time for that.
Instead, we want to find the best ways to help those suffering from illnesses like cancer. It is through reducing suffering and overcoming health challenges that we truly improve the world.
About the Author:
Donald R. Yance is the founder of the Mederi Center. A Clinical Master Herbalist and Certified Nutritionist, Donnie is renowned for his extraordinary knowledge and deep understanding of the healing properties of plants and nutrition, as well as of epigenetics, laboratory medicine, oncologic pathology, and molecular oncology. He is a professional member of the American Herbalists Guild, National Association of Nutrition Professionals, Academy of Integrative Health and Medicine, and the Society for Integrative Oncology.
[1] Jin, J., Byun, JK., Choi, YK. et al. Targeting glutamine metabolism as a therapeutic strategy for cancer. Exp Mol Med 55, 706–715 (2023). https://doi.org/10.1038/s12276-023-00971-9
[2] Bo Li and M. Celeste Simon, Molecular Pathways: Targeting MYC-induced Metabolic Reprogramming and Oncogenic Stress in Cancer, Clin Cancer Res. 2013 November 1; 19(21): . doi:10.1158/1078-0432.CCR-12-3629
[3] Zamani P, Matbou Riahi M, Momtazi-Borojeni AA, Jamialahmadi K. Gankyrin: a novel promising therapeutic target for hepatocellular carcinoma. Artif Cells Nanomed Biotechnol. 2018 Nov;46(7):1301-1313. doi: 10.1080/21691401.2017.1388250. Epub 2017 Oct 12. PMID: 29025272.
[4] Liu R, Li Y, Tian L, Shi H, Wang J, Liang Y, Sun B, Wang S, Zhou M, Wu L, Nie J, Lin B, Tang S, Zhang Y, Wang G, Zhang C, Han J, Xu B, Liu L, Gong K, Zheng T. Gankyrin drives metabolic reprogramming to promote tumorigenesis, metastasis and drug resistance through activating β-catenin/c-Myc signaling in human hepatocellular carcinoma. Cancer Lett. 2019 Feb 28;443:34-46. doi: 10.1016/j.canlet.2018.11.030. Epub 2018 Nov 29. PMID: 30503555.
[5] Kiyoung Eun, Seok Won Ham, Hyunggee Kim, Cancer stem cell heterogeneity: origin and new perspectives on CSC targeting, BMB Rep. 2017; 50(3): 117-125
[6] Jhudit Perez-Escuredo, Rajesh K Dadhich, Suveera Dhup, Andrea Cacace, Vincent F Van Hee, Christophe J De Saedeleer, Martina Sboarina, Fabien Rodriguez, Marie-Josephine Fontenille, Lucie Brisson, Paolo E Porporato, and Pierre Sonveaux , Lactate promotes glutamine uptake and metabolism in oxidative cancer cells, CELL CYCLE 2016, VOL. 15, NO. 1, 72–83 http://dx.doi.org/10.1080/15384101.2015.1120930
[7] Sidoryk M, Matyja E, Dybel A, Zielinska M, Bogucki J, Jaskolski DJ, et al. Increased expression of a glutamine transporter SNAT3 is a marker of malignant gliomas. Neuroreport. 2004; 15: 575-8.
[8] Dolinska M, Dybel A, Zablocka B, Albrecht J. Glutamine transport in C6 glioma cells shows ASCT2 system characteristics. Neurochem Int. 2003; 43: 501-7.
[9] Ju, HQ., Lin, JF., Tian, T. et al. NADPH homeostasis in cancer: functions, mechanisms and therapeutic implications. Sig Transduct Target Ther 5, 231 (2020). https://doi.org/10.1038/s41392-020-00326-0
[10] DeBerardinis RJ, Cheng T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010; 29: 313-24.
[11] Vazquez A, Kamphorst JJ, Markert EK, Schug ZT, Tardito S, Gottlieb E. Cancer metabolism at a glance. J Cell Sci. 2016; 129: 3367-73.
[12] DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007; 104: 19345-50.
[13] Caniglia JL, Jalasutram A, Asuthkar S, Sahagun J, Park S, Ravindra A, Tsung AJ, Guda MR, Velpula KK. Beyond glucose: alternative sources of energy in glioblastoma. Theranostics. 2021 Jan 1;11(5):2048-2057. doi: 10.7150/thno.53506. PMID: 33500708; PMCID: PMC7797684.
[14] DeBerardinis RJ, Cheng T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–324. doi: 10.1038/onc.2009.358.
[15] Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340–1344. doi: 10.1126/science.1193494.
[16] Zygogianni A, Kyrgias G, Kouvaris J, Pistevou-Gombaki K, Capezzali G, Zefkili S, Kokkakis J, Georgakopoulos J, Kelekis N, Kouloulias V. Impact of acute radiation induced toxicity of glutamine administration in several hypofractionated irradiation schedules for head and neck carcinoma, Head Neck Oncol. 2012 Dec 14;4(5):86.
[17] Liu R, Li Y, Tian L, Shi H, Wang J, Liang Y, Sun B, Wang S, Zhou M, Wu L, Nie J, Lin B, Tang S, Zhang Y, Wang G, Zhang C, Han J, Xu B, Liu L, Gong K, Zheng T. Gankyrin drives metabolic reprogramming to promote tumorigenesis, metastasis and drug resistance through activating β-catenin/c-Myc signaling in human hepatocellular carcinoma. Cancer Lett. 2019 Feb 28;443:34-46. doi: 10.1016/j.canlet.2018.11.030. Epub 2018 Nov 29. PMID: 30503555.
[18] Reyhaneh Salehi-Tabar, Loan Nguyen-Yamamoto, Luz E. Tavera-Mendoza, Thomas Quail, Vassil Dimitrov, Beum-Soo An, Leon Glass, David Goltzman, and John H. White ,Vitamin D receptor as a master regulator of the c-MYC/MXD1 network, www.pnas.org/cgi/doi/10.1073/pnas.1210037109 , PNAS | November 13, 2012 | vol. 109 | no. 46 | 18827–18832
[19] Huanjie Yang, Ying Wang, […], and Anil Wali, Withaferin A Inhibits the Proteasome Activity in Mesothelioma In Vitro and In Vivo, PLoS One. 2013 February 8; 8(2): 10.1371/annotation/1f7766a6-35da-4d34-b07b-4c06667bdbec.
[20] Huang HL, Weng HY, Wang LQ, Yu CH, Huang QJ, Zhao PP, Wen JZ, Zhou H, Qu LH. Triggering Fbw7-mediated proteasomal degradation of c-Myc by oridonin induces cell growth inhibition and apoptosis. Mol Cancer Ther. 2012 May;11(5):1155-65. doi: 10.1158/1535-7163.MCT-12-0066. Epub 2012 Mar 2. PMID: 22389469.
[21] Wang Y, Lv H, Dai C, Wang X, Yin Y, Chen Z. Oridonin Dose-Dependently Modulates the Cell Senescence and Apoptosis of Gastric Cancer Cells. Evid Based Complement Alternat Med. 2021 Nov 9;2021:5023536. doi: 10.1155/2021/5023536. PMID: 34795783; PMCID: PMC8595004.
[22] Elbadawi M, Boulos JC, Dawood M, Zhou M, Gul W, ElSohly MA, Klauck SM, Efferth T. The Novel Artemisinin Dimer Isoniazide ELI-XXIII-98-2 Induces c-MYC Inhibition, DNA Damage, and Autophagy in Leukemia Cells. Pharmaceutics. 2023 Mar 30;15(4):1107. doi: 10.3390/pharmaceutics15041107. PMID: 37111592; PMCID: PMC10144546.
[23] Minting Lin, Hong B, et. al., Parthenolide suppresses non-small cell lung cancer GLC-82 cells growth via B-Raf/MAPK/Erk pathway, Oncotarget, 2017, Vol 8, (No. 14), pp: 23436-23447
[24] Bo Li and M. Celeste Simon, Molecular Pathways: Targeting MYC-induced Metabolic Reprogramming and Oncogenic Stress in Cancer, Clin Cancer Res. 2013 November 1; 19(21): . doi:10.1158/1078-0432.CCR-12-3629.
[25] RamakrishnaVadde, SridharRadhakrishnanaHewageEranda Karunathilake KurunduaLavanyaReddivaribJairam K.P.Vanamalaac Indian gooseberry (Emblica officinalisGaertn.) suppresses cell proliferation and induces apoptosis in human colon cancer stem cells independent of p53 status via suppression of c-Myc and cyclin D1, Journal of Functional Foods, Volume 25, August 2016, Pages 267-278
[26] Sivalokanathan Sarveswaran, Ritisha Ghosh, Rujul Parikh, and Jagadananda Ghosh, Wedelolactone, an anti-inflammatory botanical, interrupts c-Myc oncogenic signaling and synergizes with enzalutamide to induce apoptosis in prostate cancer cells, Mol Cancer Ther. 2016 November ; 15(11): 2791–2801. doi:10.1158/1535-7163.MCT-15-0861.


Thanks doc. The glutamine issue is one that’a being on my mind for several months now. First I used it with my patients (I’m a functional/integrative MD in Mexico city) then stopped, now thanks to you I’m using it again.